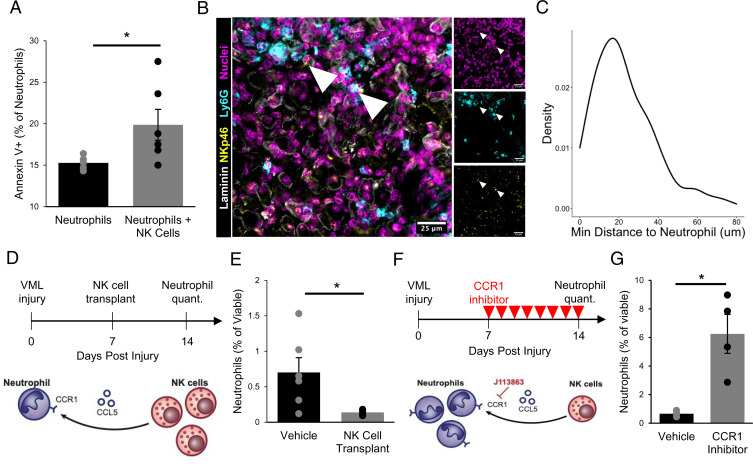
Fig. 4.
NK cells interact with neutrophils in VML injuries. (A) NK cells induce neutrophil apoptosis in coculture based on increased annexin V+ cells, as analyzed by flow cytometry. *P < 0.05. n = 5 to 6 wells. Each well contains a pool of neutrophils that are FACS-enriched from four 3-mm VML defects 7 dpi. NK cells were MACS-enriched from one spleen and activated in vitro for 48 h. Bars show mean ± SEM. (B) Immunofluorescent stains for DAPI, NKp46, Ly6G, and laminin in VML defects 7 dpi show colocalization of neutrophils and NK cells. (Scale bar, 25 μm.) (C) Distributions of minimum Euclidean distances between NK cells; 92.5% of NK cells are within 50 μm of the nearest neutrophil. n = 159 NK cells and 397 neutrophils from nine 60× images of four defects. (D) Schematic whereby 50,000 in vitro activated NK cells were transplanted into degenerative (3-mm) defects at 7 dpi. Contralateral control limbs received PBS injections. Neutrophils were quantified at 14 dpi using flow cytometry (CD11b+Ly6g+). (E) NK cell transplant significantly reduced neutrophil abundance. *P < 0.05 by two-sided, two-sample t test. Cohen’s d = 1.56. n = 5 to 6 muscles (n = 6 mice), repeated twice. Graph shows mean ± SEM. (F) Schematic of experiment design whereby a cohort of mice received degenerative (3-mm) defects followed by daily intraperitoneal injection of CCR1 inhibitor (J113863) between 7 and 14 dpi. Neutrophil populations were quantified by flow cytometry (CD11b+Ly6g+) at 14 dpi. (G) CCR1 inhibition significantly increased neutrophil abundance in 3-mm defects, to a similar extent as the NK cell transplant reduced neutrophil populations, suggesting that NK cells may locally recruit neutrophils via CCL5 secretion to induce contact-dependent apoptosis. *P < 0.05 by two-sided, two-sample t test. n = 3 to 4 mice per group, repeated twice. Cohen’s d = 2.64. Graph shows mean ± SEM.