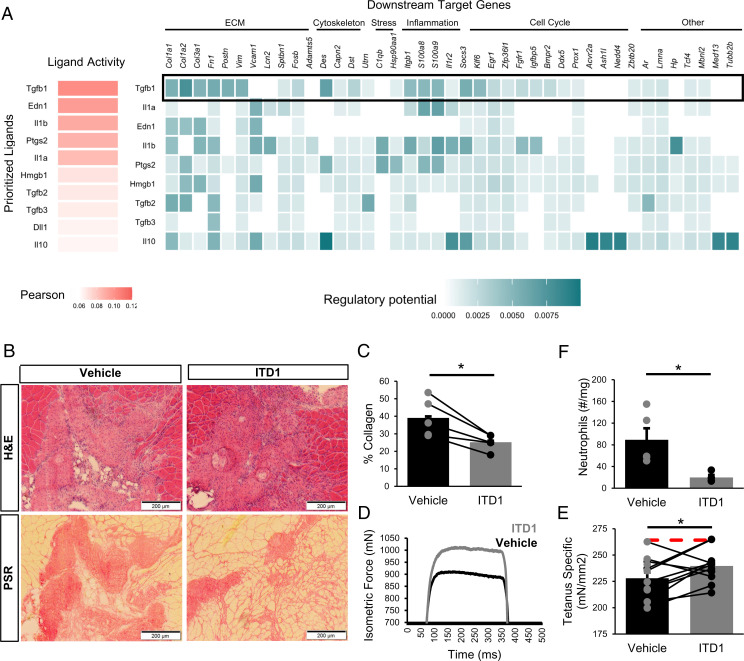
Fig. 6.
Inhibition of TGFβ signaling partially restores muscle regeneration and reduces fibrosis after VML injury. (A) NicheNet ranking of the ligands that best predict MuSC differential gene expression at 7 dpi. Pearson correlation coefficient indicates the ability of each ligand to predict the expression of differentially expressed genes. TGFβ1 is predicted to be a key contributor to MuSC dysfunction. (B) Representative H&E and PSR images from 3-mm defects 28 dpi following intramuscular treatment with ITD1 (a TGFβ-signaling inhibitor) or vehicle every 3 d until 15 dpi. (C) Defects treated with ITD1 showed reduced collagen deposition. Collagen was calculated as a percentage of the 10× magnification image of the defect that was stained red by PSR. n = 4 tissues per condition (n = 4 mice), where contralateral limbs were treated with vehicle. *P < 0.05 by two-sided, paired t test. Bars show mean ± SEM. Cohen’s d = 1.83. (D) Representative force curves for muscles treated with ITD1 or vehicle. (E) Specific force following muscle stimulation of injured TA muscles 28 dpi improved by ITD1 treatment. Bars show mean ± SEM, and red dashed line indicates specific force of uninjured, untreated TA muscles. *P = 0.023 by two-sided, paired t test. n = 10 muscles (n = 10 mice), Cohen’s d = 1.1. (F) Neutrophil abundance following ITD1 treatment, as quantified by flow cytometry 14 dpi, shows that TGFβ signaling inhibition reduces neutrophil recruitment. Boxplots show mean ± SEM. n = 4 defects (n = 4 mice). *P = 0.025 by two-sided, two-sample t test. Cohen’s d = 1.91.