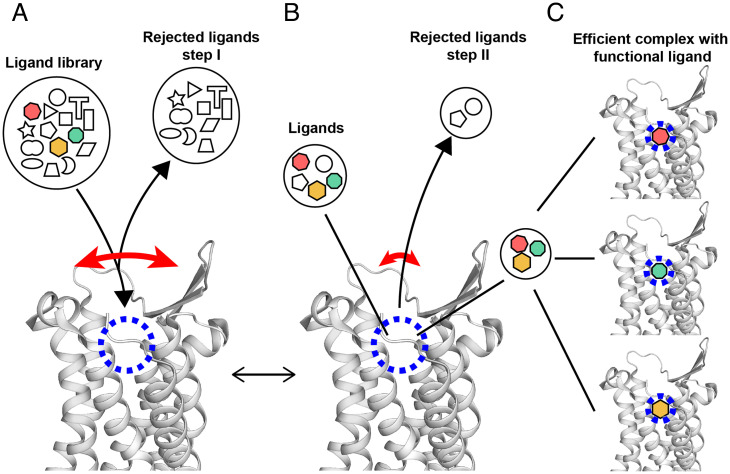
Fig. 4.
Hypothetical multistep selection of functional orthosteric ligands by GPCRs based on the NMR observation of transient large-amplitude structure fluctuations and observations with crystallization assays (39). A side-view of the extracellular part of the NK1R crystal structure (PDB ID code 6J20) is shown. The long curved red arrow indicates large transient openings of the ligand-binding groove. The short red arrow indicates a closed conformation of the GPCR, which is populated most of the time. The blue dashed circle indicates the location in the binding groove where ligands interact with specific amino acid residues of the GPCR. The black lines refer symbols to locations in the NK1R structure. Black arrowheads indicate flows of ligands leading to equilibrium at given concentrations of NK1R and ligands, solution conditions and temperature. (A) Initial screening of ligands by size and shape. (B) Selection of high-affinity ligands within the binding groove. (C) Different functional ligands are specifically bound to the receptor, yielding different functional states. Depending on the availability of such, ligands in the solution milieu (colored symbols in A) and their relative binding affinities, one of the ligands may be predominantly bound and imprint its efficacy on GPCR.