Figure 9.
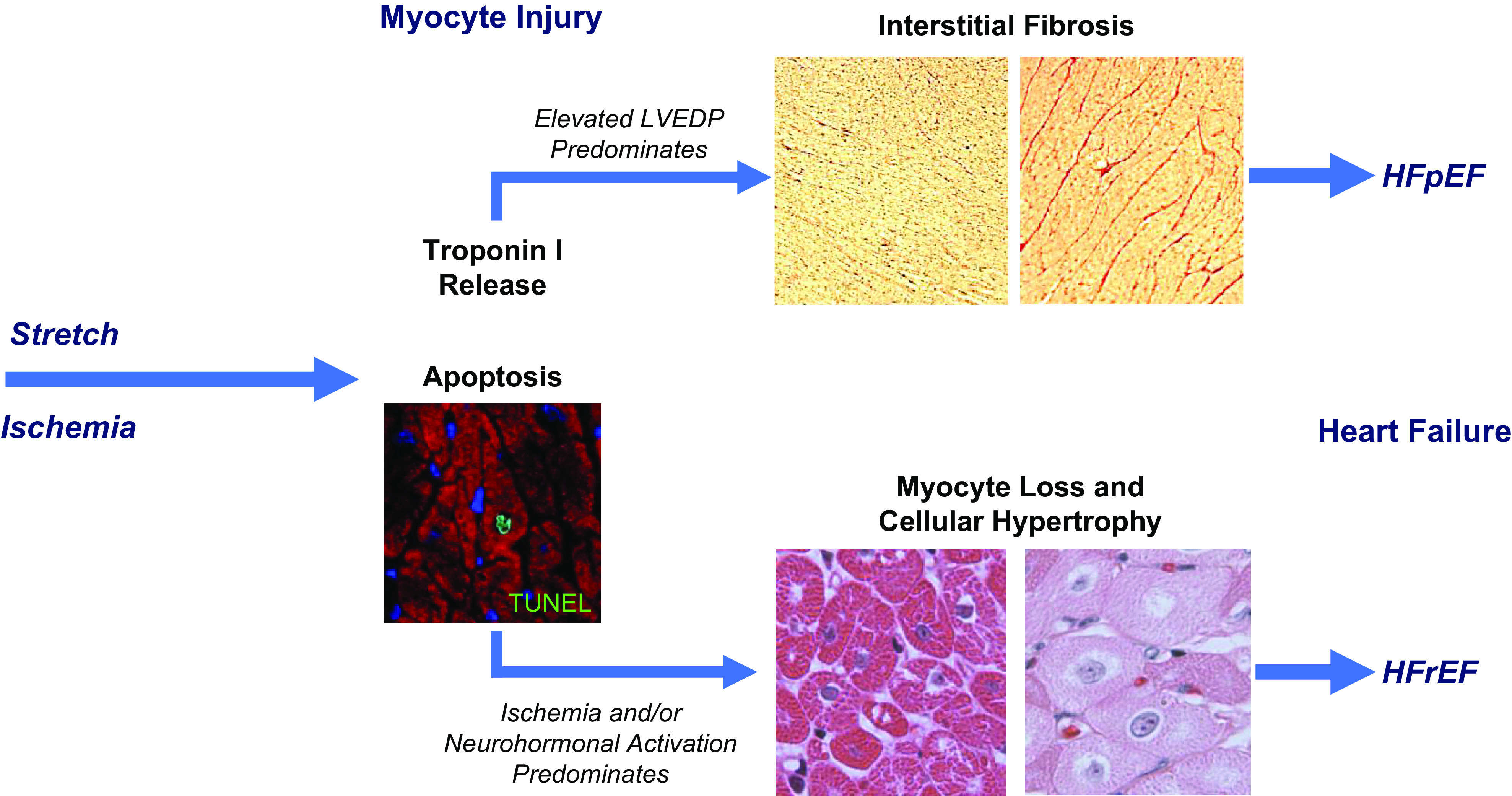
Differential effects of ischemia and preload-induced myocardial injury on the functional phenotype of heart failure. Preload elevation and ischemia both lead to troponin release in association with myocyte apoptosis by TUNEL staining. While single episodes are reversible, repetitive injury from either stress leads to myocyte loss. In the case of preload elevation, the diastolic strain also results in marked interstitial fibrosis. This may arise as a consequence of an increased inflammatory response to myocyte injury or activation of myofibroblasts in response to mechanical stimuli. The development of fibrosis prevents progressive myocyte loss but results in a physiological phenotype of HFpEF with concentric left ventricular (LV) remodeling and reduced LV compliance. Over long periods of time, labile systolic hypertension, reduced aortic compliance, and aging likely contribute to episodic preload elevation in humans. In contrast, the development of apoptosis in response to repetitive nontransmural ischemia appears to be insufficient to elicit a robust fibrotic response. As a result, myocyte loss persists and cellular hypertrophy predominates. When this impacts a large amount of viable myocardium or is associated with irreversible injury from a myocardial infarction, global LV function deteriorates and neurohormonal activation leads to further myocyte injury and loss throughout the heart. Over time, this leads to left ventricular dilatation and ischemic cardiomyopathy with a phenotype of HFrEF. HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction.
