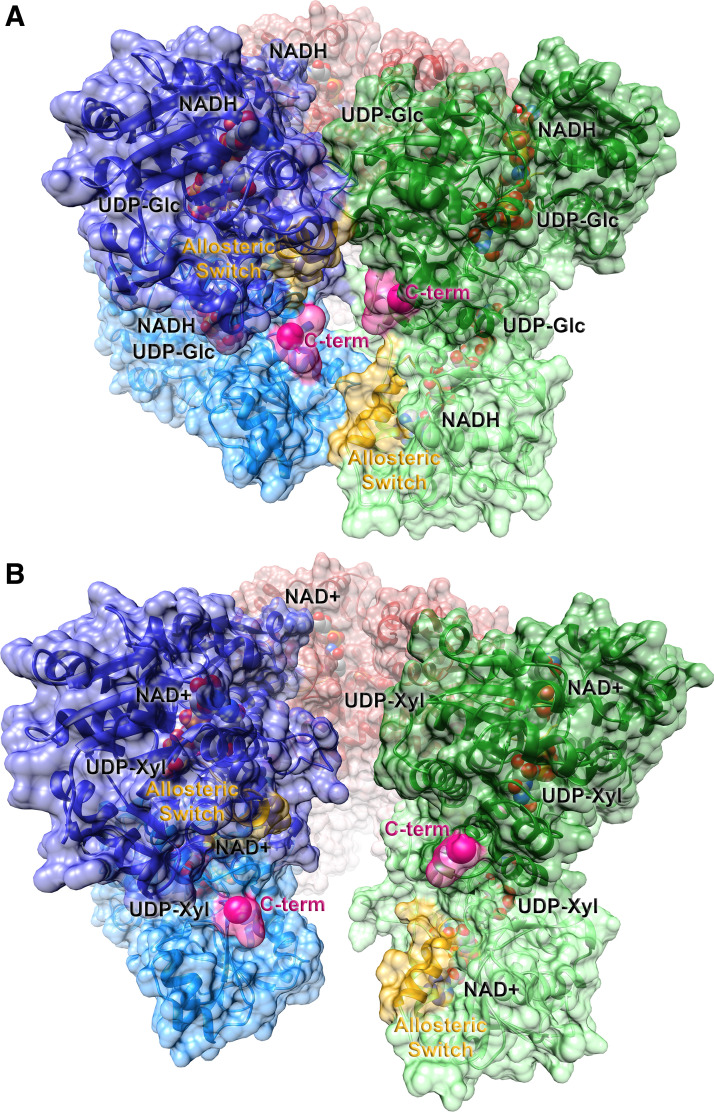
Figure 2.
Ribbon representations of the active (E) and inhibited (EΩ) substate conformations of hexameric human UGDH. The abortive ternary complex of the substrate, UDP-glucose, and the reduced cofactor, NADH [2Q3E (26); A] and the feedback inhibited complex of UDP-xylose and NAD+ [3PTZ (27); B] are shown in ribbon representation. The solvent accessible surface representation is superimposed. Individual dimers are illustrated in dark/light pairs of red, green, and blue. The allosteric switch, which includes the Thr 131 loop and the adjacent α6 helix, is labeled and highlighted in yellow; the start of the intrinsically disordered C-terminal tail is labeled and colored in pink. Ligands are shown in space-filling representation with oxygen colored in red, nitrogen in blue, carbon in gray, and phosphorus in orange. UGDH, UDP-glucose dehydrogenase.