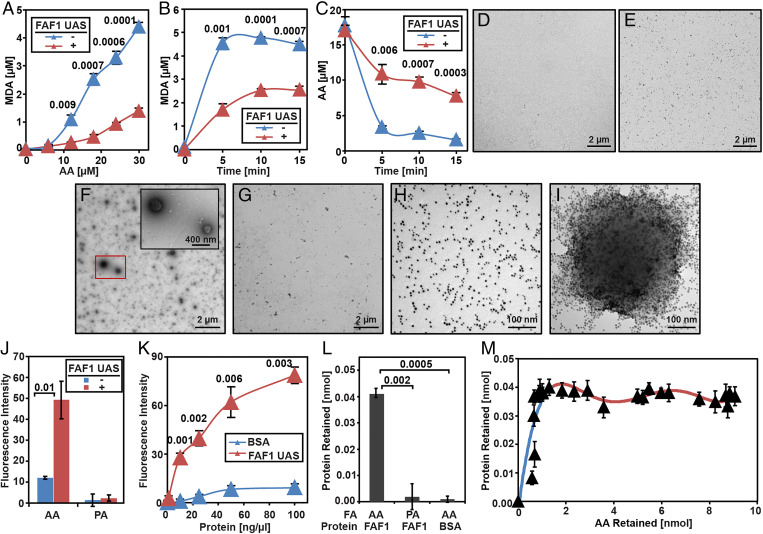
Fig. 4.
Purified FAF1 sequesters AA from iron-catalyzed peroxidation through the UAS domain. (A–C) Amount of MDA produced (A and B) or AA remained (C) in reactions containing AA (A, indicated amount; B, 30 µM), 50 µM H2O2 and 100 µM FeSO4 with or without 0.3 mg/mL purified UAS domain of FAF1 for 5 min (A) or indicated time (B and C). (D–I) Negative staining EM images of AA (30 µM) (D), UAS domain of FAF1 (0.1 mg/mL) (E), AA mixed with the UAS domain (F), or BSA (0.1 mg/mL) (G), and gold-labeled UAS domain in the absence (H) or presence (I) of AA. (J and K) Nile Red fluorescence of solutions containing 30 µM AA (J and K) or PA (J) and the protein (0.1 mg/mL, indicated concentration in K). (L) Amount of the protein retained by filtration of solutions containing indicated proteins (50 µg/mL) and FAs (50 µM). (M) Amount of the UAS domain plotted against that of AA retained by the filtration of solutions containing 50 µg/mL UAS domain and 0 to 400 µM AA. (A–C and J–M) Results are reported as mean ± SEM from three independent experiments. Statistical significance was determined by unpaired, two-tailed t test.