Table 2.
Control Experiments Establishing an Acid-Catalyzed Mechanism for the Rearrangement of Furan 5b to Indole 6
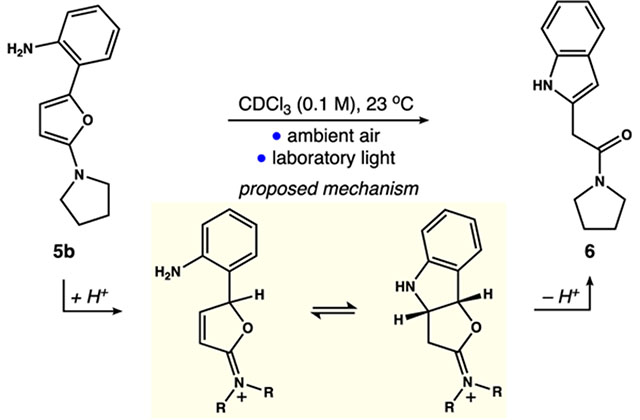
| |||
|---|---|---|---|
| entry | modificationa | time | NMR yield of 6(%)b |
| 1 | none | 6 h | 86 |
| 2 | hν = 350 nm | 5 min | 84 |
| 3 | no light | 6 h | trace |
| 4 | 0.5 equiv of K2CO3 | 6 h | trace |
| 5 | no light, 3 mol % of Rh2(esp)2 | 6 h | 27 |
| 6 | no light, 10 mol % of TsOH | 1 h | 89 |
No light = protected from light with aluminum foil.
Yields determined by 1H NMR (400 MHz) using bibenzyl as an internal standard.
