Table 1:
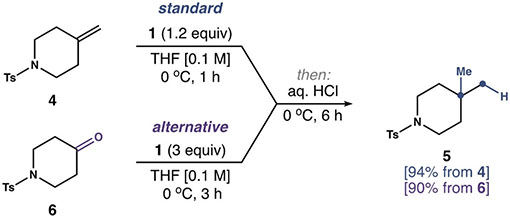
| |||
|---|---|---|---|
|
| |||
| Entry | Deviation from standard procedure | t [h] | Yield 5 [%] |
| 1 | PhMe, 1 equiv DMAP | 6 | 16[d] |
| 2 | PhMe | 6 | 0 |
| 3 | PhMe/THF (2:1) | 2 | 80 |
| 4 | none | 1 | 94 |
| 5 | TFA as proton source[e] | 1 | 92 |
| 6 | none, gram scale | 1 | 89 |
| 7 | commercial solution of 1[f] | 6 | 70[d] |
Yields are based on isolated 5.
Reactions were carried out on a 0.2 mmol scale.
Reagent 1 was prepared directly before use.[25]
Reflects conversion of 4 into 5 as judged by 1H NMR spectroscopic analysis of the unpurified reaction mixture.
The reaction was treated with TFA at −78°C and warmed to rt.
Commercial 1 at 0.52 m (in PhMe) was used as received.
