Abstract
Publicly funded research has contributed enormously to many products that were developed in the face of the COVID-19 pandemic. Yet universities' technology transfer practices have failed to ensure that these products are available in low- and middle-income settings. Drawing upon the example of the lipid nanoparticle delivery technology – which was developed in and around the University of British Columbia in Vancouver, BC, and incorporated into the Pfizer/BioNTech COVID-19 vaccine – we show the divide between the university's stated principles to serve global health and technology transfer in practice. We outline three policy actions to realign universities' technology transfer practices in the service of global health.
Abstract
La recherche financée par l'État a grandement contribué au développement de nombreux produits face à la pandémie de la COVID-19. Pourtant, les pratiques de transfert de technologie des universités n'ont pas réussi à garantir que ces produits soient disponibles dans les pays à revenu faible ou intermédiaire. L'exemple de la technologie à nanoparticules lipidiques – qui a été développée entre autre par l'Université de la Colombie-Britannique, à Vancouver, et incorporée au vaccin Pfizer/BioNTech contre la COVID-19 – montre le fossé entre, d'une part, les principes énoncés par l'université au service de la santé mondiale et, d'autre part, le transfert de technologie dans la pratique. Nous décrivons trois actions politiques pour réaligner les pratiques de transfert de technologie des universités au service de la santé mondiale.
Introduction
While Canada has imported more doses from Pfizer/BioNTech than from any other COVID-19 vaccine makers, many may be unaware that a crucial component of the mRNA vaccine was developed domestically. The vaccine incorporates a “lipid nanoparticle” (LNP) delivery system that was invented and developed by researchers embedded in a web of biotechnology companies (Box 1), which sprang out of the University of British Columbia (UBC) (Dolgin 2021; Vardi 2021) in Vancouver, BC. It is uncertain as to who controls the technology. The validity of some of the patents pertaining to the LNP technology remains the subject of an ongoing legal dispute between one of UBC's spin-off companies (Arbutus Biopharma) and the American biopharmaceutical company Moderna, Inc., which in collaboration with the National Institutes of Health (NIH) in the US, produced the other COVID-19 mRNA vaccine (“Moderna Loses Key Patent” 2020). But without the LNP delivery system, Pfizer/BioNTech's life-saving vaccine (and likely also the NIH/Moderna mRNA vaccine [Vardi 2021]) would not work (Buschmann et al. 2021).
Box 1. Developing the LNP delivery system: A complex backstory.
The story behind the development of the LNP delivery system is complex. According to patent filings, the LNP technology was originally invented in the mid-2000s by several scientists, including Ian MacLachlan, who were then employed at Protiva Biotherapeutics. Understanding who controls the LNP delivery system and what products it has been integrated into is, however, clouded by an array of corporate transactions, trade secrecy, regulatory rules and multiple rounds of litigation.
The litigation mainly appears to be the product of interpersonal rivalry and corporate manoeuvring. Protiva was spun out of another company, Inex Pharmaceuticals (later renamed Tekmira), which had been co-founded by UBC biochemist Pieter Cullis in the 1990s (considered a pioneer in the field of LNPs and other technologies, Cullis has been involved in multiple companies' efforts to commercialize promising therapeutics). As MacLachlan (through Protiva) pursued the development of a gene therapy with a Massachusetts-based company, Alnylam Pharmaceuticals Inc., demonstrating the first effective “gene silencing” therapy in monkeys using the LNP system, his former Inex colleagueturned rival, Thomas Madden, vied for Alnylam's attention. Lawsuits followed, eventually resulting in Alnylam assigning ownership of the LNP patents back to Protiva/Tekmira and paying $65 million to settle the case. Importantly, the biotechnology company AlCana Therapeutics (created by Madden and Cullis), which later became Acuitas Therapeutics, was also granted a licence under the 2012 settlement to utilize MacLachlan's LNP delivery system for the purposes of developing novel mRNA products. In 2015, AlCana/Acuitas sub-licensed the LNP technology to Moderna, Inc., for the development of an mRNA influenza vaccine, precipitating another round of litigation between Tekmira (then renamed Arbutus Biopharma) and Acuitas. As part of a new settlement in 2018, Acuitas' licence to the LNP technology was terminated. But the core LNP technology, through various partnerships, appears to be embedded in a range of products, including a regulatory-approved rare disease gene therapy, mRNA-based cancer treatments currently in development and at least one COVID 19 vaccine. Arbutus and Moderna meanwhile continue to dispute their respective patent rights before the US Patent Trial and Appeal Board.
Moderna has indicated it will not enforce any COVID–19 patent rights during the pandemic. But neither Arbutus (the owner of MacLachlan's patents on the LNP system) nor Acuitas (which worked with Pfizer/BioNTech to develop its COVID–19 vaccine delivery technology) has signalled the same forbearance. None of these companies has shared the precise details of the LNP delivery system that is incorporated into the mRNA COVID–19 vaccines. Arbutus' LNP delivery system patents have been filed in South Africa, India and China, among other places, where key would-be producers of COVID–19 vaccines have thus far struggled to re-engineer the underlying LNP technology.
The information contained in Box 1 derives from multiple sources (Aizenman 2021; Akinc et al. 2019; Dolgin 2021; Vardi 2021; Zimmermann et al. 2006), including patent applications pertaining to the LNP delivery system (Yaworski et al. 2011, 2016
Against a background of an insufficient supply of COVID-19 vaccines to meet global need and vaccine-makers resisting efforts to share the underlying knowledge (Furlong 2021), we trace how the LNP technology came to be privately controlled. In the past, observers lauded UBC for its global health–oriented approach to technology transfer, which focused on licensing biomedical discoveries in ways that promote access in low- and middle-income countries (LMICs) (“University Global Health Impact” n.d.). However, the COVID-19 pandemic reveals a departure from this goal and a gap between technology transfer in principle and technology transfer in practice. We focus on this gap in order to motivate policy change.
Technology Transfer and Global Health
Technology transfer encapsulates a range of activities designed to move a discovery from “bench to bedside.” The predominant approach to technology transfer relies upon intellectual property (IP), especially patents (Table 1), as a tool to attract investment and create agreements between two or more parties, all with an intention of spurring follow-on research (Burk and Lemley 2003; Herder et al. 2020). The process can consume many years spanning discovery through pre-clinical research, clinical trials involving humans and, eventually, regulatory approval. Most drugs and vaccines fail along this path, but the process often begins with a discovery in a university lab, which is patented and transferred into a newly formed “spin-off” company, hoping to attract partners and investment on the strength of its IP position.
Table 1.
A glossary of IP and related legal instruments
| Type of IP | Description | Duration | Example |
|---|---|---|---|
| Patents | A set of exclusive rights to use, make, sell and import an “invention” that must be applied for and, if the criteria of the Patent Act (1985) are met, granted; patent rights are country- or regionspecific | 20 (or more) years from the date of filing a patent application with the potential for extensions due to regulatory delays | A novel lipid-nucleic acid particulate complex that is useful for in vitro or in vivo gene transfer |
| Trade secrets and confidential business information (CBI) | An exclusive right pertaining to information, scientific or technical, with respect to trade secrets and business in the case of CBI, that is valuable to its owner due to its secrecy and which its holder has taken reasonable steps to keep confidential; no application is required | Unlimited unless the information is no longer kept secret or is independently created. In addition, CBI protection is unavailable if the regulator deems it no longer to be CBI under the Food and Drug Regulations (2022) in Canada | Trade secrets include information pertaining to vaccine manufacturing processes – that is, manufacturing “know-how”, while CBI includes unpublished clinical trial results |
| IP assignments, patent licences and cross-licences, research collaboration agreements, etc. | Contractual agreements that involve the transfer of IP or granting another party permission to use IP rights (whether patents, trade secrets and/or CBI) | The term specified by the parties to the contractual agreement | A licensed agreement granting permission to use patented LNP technology for the development of gene therapies |
None of these forms of IP are mutually exclusive from one another; rather, in practice, actors often utilize these diverse forms of IP and IP-related contracting in conjunction with one another.
Many drugs and vaccines in use today, especially those that are important for public health, emanate from publicly funded environments (Herder et al. 2020). This does not necessarily mean that technology transfer is functioning optimally. On the contrary, there are several instances where the current, IP-focused approach to technology transfer has slowed or stifled research. Gene patenting is a classic, but contested, example (Bubela et al. 2015). Other IP rights, such as contracts that treat research data as trade secrets, can also be detrimental to knowledge translation (Williams 2013). Even when a useful product is invented, access to it by populations in need, particularly in LMICs, may be limited by IP, high product pricing, or both (Padmanabhan et al. 2010).
Scientists at Yale University, Connecticut, US, developed d4T – one of the historically most important therapies for HIV/AIDS. By 2001, d4T had been licensed by the university's technology transfer office on an exclusive basis to the American biopharmaceutical company Bristol Myers Squibb, which priced the drug in a way to render it inaccessible to millions suffering with HIV/AIDS in much of the world (Kapczynski et al. 2005). Through concerted advocacy efforts, however, Bristol Myers Squibb eventually allowed for generic production of d4T by a South African company, triggering a 30-fold reduction in price and huge scale-up in global access.
Building upon this success, a global movement in favour of open access to university-generated health products coalesced. UBC was the Canadian leader in this effort, creating a set of “Global Access Principles” in 2007 (Wasan et al. 2009) that directly informed how it licensed a low-cost, oral formulation of amphotericin B – a novel treatment for leishmaniasis that nearly exclusively occurs in LMICs (Chen et al. 2010). How often these principles were actually implemented into technology transfer practices is not known, however, because technology transfer activities are typically kept confidential, and universities do not track their adherence to these principles. But it is apparent that without access to the LNP delivery system incorporated into the mRNA vaccines, would-be LMIC-based manufacturers are struggling to produce their own COVID-19 vaccines (Aizenman 2021).
The Development of the LNP Delivery System at the University–Industry Boundary
The LNP delivery system emerged from decades of research in the area of lipids led in significant part by UBC's Pieter Cullis (Dolgin 2021). Throughout the process, Cullis raised millions in federal government funding to support his research while also founding a number of companies to commercialize his findings, including Inex Pharmaceuticals (later becoming Tekmira and known today as Arbutus Biopharma) as well as Acuitas, which worked with Pfizer/BioNTech in developing its COVID-19 vaccine (Box 1). Along the way, dozens of patents were filed by UBC, Arbutus, Acuitas and a host of other entities pertaining to the LNP technology (Gaviria and Kilic 2021). Consistent with the assumption that IP spurs commercialization, IP licensing agreements between Arbutus, Acuitas and other companies in the field that Cullis did not help form, such as the US-based Alnylam, also fuelled a number of research partnerships (Leung et al. 2019).
However, a significant amount of litigation also attended these efforts to commercialize the LNP technology (Box 1). According to an investigation by -Forbes magazine, Cullis, Madden and MacLachlan – all of whom once worked together at Inex – became embroiled in a series of lawsuits among Arbutus, Acuitas and Alnylam, alleging misappropriation of trade secrets and/or patent infringement (Vardi 2021). Arbutus (the holder of several LNP patents) is still in the midst of a patent dispute with Moderna (Brennan 2021; Gaviria and Kilic 2021; “Moderna Loses Key Patent” 2020). New products incorporating the LNP technology, including but not limited to COVID-19 vaccines, have since entered the market (Wan et al. 2014). Yet it is far from clear whether this complicated web of patent rights, contractual agreements, spin-off companies, corporate reorganizations and litigation ultimately hastened or complicated the development of products using LNP technology (Figure 1).
Figure 1.
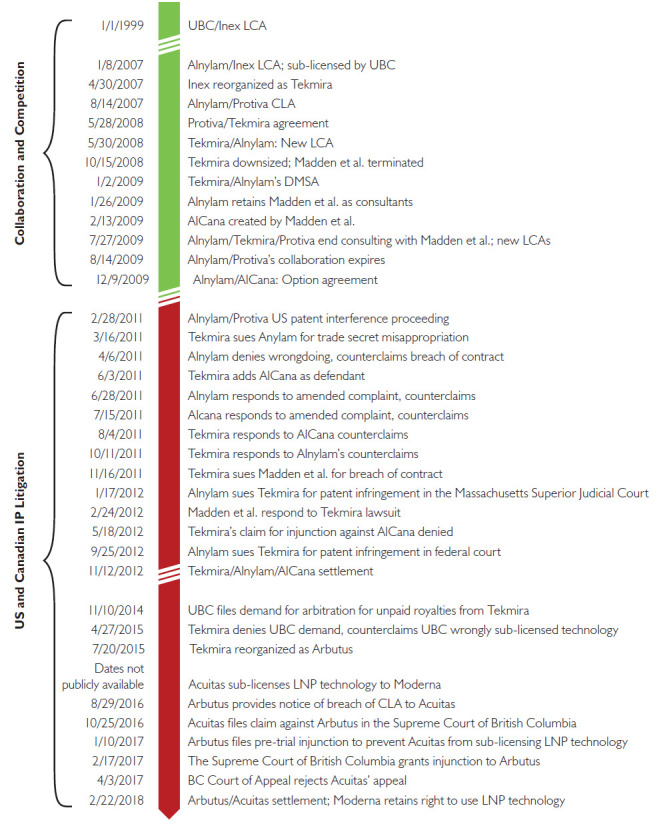
Collaboration, competition and litigation related to LNP technology
CLA = cross-license agreement; DMSA = development, manufacturing, supply agreement.; LCA = license and collaboration agreement; Option agreement = an agreement that confers upon one or more parties the right to renew an existing agreement.
A number of the companies mentioned in this figure underwent corporate reorganizations during the time-frame depicted: Inex was reorganized as Tekmira and then later as Arbutus; Protiva was a subsidiary of Tekmira; and AlCana subsequently became Acuitas.
All of the information depicted in this figure was derived from publicly available information on the website of the US Securities and Exchange Commission (https://www.sec.gov/).
It is equally unclear whether UBC's 2007 Global Access Principles had any effect on how the university managed the patents it filed related to the LNP technology, much less the IP-mediated agreements that UBC, Arbutus (which through its previously owned subsidiary Protiva filed several LNP patents starting in 2008) or Acuitas (which enjoyed a licence to use those patent rights between 2012 and 2018 and evidently still possesses relevant knowledge) struck to develop a range of products, including the agreements with Pfizer/BioNTech and Moderna for the purposes of developing COVID-19 vaccines.
Throughout the pandemic, UBC has emphasized its commitment to “mobilize COVID-19 related technology through time-limited, non-exclusive royalty-free licences, in exchange for the licensee's commitment to rapidly make and broadly distribute products and services to prevent, diagnose, treat and contain COVID-19” (UBC University–Industry Liaison Office n.d.). But it has clearly taken a hands-off approach to how UBC-founded companies manage the IP generated after they are spun off from the university, even when UBC-affiliated scientists help run those companies and when the IP is critically important to global health. There is no indication that provisions designed to allow the use of the technology in LMICs or share the underlying know-how in order to address a public health emergency were integrated into any of the IP-related agreements among UBC, Arbutus, Acuitas, Alnylam, Pfizer/BioNTech, Moderna, CureVac (a German biopharmaceutical company) and others (Gaviria and Kilic 2021). Whether LMIC-based vaccine manufacturers have the freedom to utilize the LNP technology without risk of liability for patent infringement is also unclear in the absence of greater transparency about where the various actors involved have sought patent protection.
Systemic Barriers to Realizing Global Access Commitments
The fact that UBC's stated commitment to global health has not been realized vis-à-vis the LNP technology is not its fault alone. There are multiple interconnected barriers to systemic change.
To begin with, enforcement of measures such as UBC's Global Access Principles is challenging. Those principles will only extend to sub-licences and corporate subsidiaries if UBC remains actively engaged in monitoring and enforcing compliance in subsequent commercial transactions. Universities are not always well positioned (in terms of resources) or willing (for fear of discouraging other industrial partners) to actually enforce their agreements, especially in the face of a complex array of private – demonstrably litigious and well-resourced – actors, as in the case of the LNP technology.
Achieving global access also requires overcoming multiple layers of IP protection. Even if UBC's principles had been enforced by UBC or followed by the companies connected to the university, licensing the LNP delivery system in line with the goal of improving access in LMICs would not, by itself, have resulted in the availability of an mRNA vaccine made by, or for, LMIC-based manufacturers. Access to the knowledge related to the LNP technology (in addition to those both Arbutus and Acuitas possess) and the manufacturing processes used to make COVID-19 vaccines is also essential to scaling up the production of vaccines (Erfani et al. 2021). But that know-how is treated as proprietary information and kept confidential by a range of actors, including national regulatory agencies, such that outsiders cannot discern what precise LNP formulation is in use or whether the LNP technology within the Pfizer/BioNTech and NIH/Moderna vaccines is one and the same (Vardi 2021).
Fundamentally, there exists a deep-seated deference to market actors regarding which product leads to pursue and how best to manage biopharmaceutical IP. Research funding bodies typically do not assert any interest in IP generated with public funds. The massive amounts of public funding allocated toward research and development of COVID-19 health products provided an important opportunity to reset expectations. Yet no strings were attached to any of those public dollars to ensure that the resulting products (and/or associated IP) are available to people around the world despite the need (Herder 2021). Notwithstanding the “vaccine apartheid” (Brown 2021) that has segregated LMICs from the rest of the world during the COVID-19 pandemic, efforts to establish an “IP waiver,” whereby countries loosening IP requirements over vaccines and other COVID-19 health products can do so without fear of trade sanctions, have so far failed (Krishtel and Malpani 2021).
While it may not alter the course of the current pandemic, policy action is needed to alter these deep-seated norms around biopharmaceutical innovation. University technology transfer practices offer a logical starting point, given that most health products originate in publicly funded science. Yet the COVID-19 pandemic has taught us that voluntary approaches, such as UBC's Global Access Principles, are inadequate for the task of ensuring equitable access to promising technologies such as the LNP delivery system.
Policy Actions to Improve Global Access to Publicly Funded Research
To improve access to university-developed health products in LMICs, the Canadian government can and should, at a minimum, undertake three policy actions:
The federal government should articulate a set of standardized terms and conditions that must be included in any and all IP agreements that flow from federally funded research. This will serve to ensure that the resulting knowledge and products can be accessed in LMICs without the prior consent of other parties to IP agreements. Precedents for such “equitable access licensing” terms and conditions already exist (Kapczynski et al. 2005), which could be readily expanded to include not only patents but also proprietary knowledge and adapted into policies for research funding bodies. UBC has, for instance, recently attracted over $18 million in federal funding to establish a “Nanomedicines Innovation Network” (https://www.nanomedicines.ca/). This funding should come with commitments to ensure equitable access to follow-on LNP technologies that emerge from the network's research.
- The government should ensure the transparency of IP agreements arising from publicly funded research. This requirement has two components:
- Firstly, all university-based research funded by the federal government should require – as a condition of funding – that a copy of all IP agreements (defined broadly to include patent licences, non-disclosure agreements, collaboration agreements, etc.) be provided without redaction to an independent body charged with auditing these agreements for compliance with the above-mentioned standardized terms and conditions designed to enhance equitable access. Provided it is equipped with the necessary resources and expertise (e.g., legal scholars, ethicists, etc.) to review such IP agreements, this body could be housed within an existing (e.g., the Secretariat on the Responsible Conduct of Research) or newly created organization in the federal government. But it is imperative that it operates at an arm's length from government research funding bodies, university administrations, academic researchers who have industry funding and/or who are commercializing a product and the industry itself – all of whom may have an interest in preserving the status quo. Agreements between government laboratories and private partners should similarly be shared with that same body for auditing. This requirement should apply immediately – not at the end of the funding period.
- Secondly, a copy of those agreements redacted only for pricing information and disclosing the IP previously held by the private party ought to be publicly released within a reasonable – yet short – period after signing to enable independent review and analysis.
- To counter the entrenched, IP-focused approach to biomedical research, the government should support open science approaches to drug and vaccine development (Gold 2021). This should take at least two forms:
- Firstly, the government should create specific funding programs aimed at those agreeing to abide by open science principles – open data, open materials, open tools, open publications and the absence of IP rights, which restrict others from using those data, materials and tools or building new products.
- Secondly, governments should create incentives for firms to engage in open science, such as new regulatory incentives that give priority to sharing. In return for placing key knowledge such as the LNP delivery system in the public domain, developers of therapies, vaccines and other products would be rewarded with a carefully crafted time-limited period of market exclusivity under the Food and Drug Regulations (2022).
Relying on current voluntary university and government technology transfer practices has left a hole in the global effort to combat COVID-19 and future health crises by failing to ensure equitable access to knowledge by LMICs. These problems are not new, and despite past calls, universities have failed to meet their stated commitment to global health. The actions we propose are a start on efforts necessary to redress this failure and realign university technology transfer with the public good.
Contributor Information
Matthew Herder, Director, Health Law Institute, Schulich School of Law; Associate Professor, Department of Pharmacology, Faculty of Medicine, Dalhousie University, Halifax, NS.
E. Richard Gold, James McGill Professor, Faculty of Law and Faculty of Medicine, McGill University, Montreal, QC.
Srinivas Murthy, Clinical Associate Professor, Faculty of Medicine, University of British Columbia, Vancouver, BC.
Conflict of Interest
Herder reported being a member of the Patented Medicine Prices Review Board, Canada's national drug price regulator, and receiving honoraria from the Board for his service.
Murthy's institution receives support from the Health Research Foundation and Innovative Medicines Canada through a research chair in pandemic preparedness research. He receives research support from the Canadian Institutes of Health Research (CIHR).
Funding
This project was supported by an external grant from the CIHR (CIHR PJT 156256). This work was also supported by Herder's recently awarded chair in Applied Public Health, which is funded by the CIHR and the Public Health Agency of Canada.
References
- Aizenman N. 2021, October 19. Moderna Won't Share Its Vaccine Recipe. WHO Has Hired an African Startup to Crack It. NPR. Retrieved January 13, 2022. <https://www.npr.org/sections/goatsandsoda/2021/10/19/1047411856/the-great-vaccine-bake-off-has-begun>.
- Akinc A., Maier M.A., Manoharan M., Fitzgerald K., Jayaraman M., Barros S. et al. 2019. The Onpattro Story and the Clinical Translation of Nanomedicines Containing Nucleic Acid-Based Drugs. Nature Nanotechnology 14(12): 1084–87. doi:10.1038/s41565-019-0591-y. [DOI] [PubMed] [Google Scholar]
- Brennan Z. 2021, December 1. Moderna Loses Latest Battle in Key Vaccine Delivery Patent Fight as Federal Appeal Falls Flat. Endpoints News. Retrieved January 13, 2022. <https://endpts.com/moderna-loses-latest-battle-in-key-vaccine-delivery-patent-fight-as-federal-appeal-falls-flat/>. [Google Scholar]
- Brown G. 2021, June 11. Vaccines for All or Vaccine Apartheid? Project Syndicate. Retrieved January 13, 2022. <https://www.project-syndicate.org/commentary/g7-must-finance-global-covid19-vaccination-drive-by-gordon-brown-2021-06>.
- Bubela T., Vishnubhakat S., Cook-Deegan R.. 2015. The Mouse That Trolled: The Long and Tortuous History of a Gene Mutation Patent That Became an Expensive Impediment to Alzheimer's Research. Journal of Law and the Biosciences 2(2): 213–62. doi:10.1093/jlb/lsv011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burk D.L., Lemley M.A.. 2003. Policy Levers in Patent Law. Virginia Law Review 89(7): 1575–96. doi:10.2307/3202360. [Google Scholar]
- Buschmann M.D., Carrasco M.J., Alishetty S., Paige M., Alameh M.G., Weissman D.. 2021. Nanomaterial Delivery Systems for mRNA Vaccines. Vaccines 9(1): 65. doi:10.3390/vaccines9010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.E., Gilliland C.T., Purcell J., Kishore S.P.. 2010. The Silent Epidemic of Exclusive University Licensing Policies on Compounds for Neglected Diseases and Beyond. PLOS Neglected Tropical Diseases 4(3): e570. doi:10.1371/journal.pntd.0000570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolgin E. 2021. The Tangled History of mRNA Vaccines. Nature 597(7876): 318–24. doi:10.1038/d41586-021-02483-w. [DOI] [PubMed] [Google Scholar]
- Erfani P., Binagwaho A., Jalloh M.J., Yunus M., Farmer P., Kerry V.. 2021. Intellectual Property Waiver for COVID-19 Vaccines Will Advance Global Health Equity. BMJ 374: n1837. doi:10.1136/bmj.n1837. [DOI] [PubMed] [Google Scholar]
- Food and Drug Regulations, C.R.C., c. 870, Food and Drugs Act. 2022, January 12. Retrieved January 25, 2022. <https://laws-lois.justice.gc.ca/PDF/C.R.C.,_c._870.pdf>.
- Furlong A. 2021, May 14. Big Vaccine Makers Reject Offers to Help Produce More Jabs. Politico. Retrieved January 13, 2022. <https://www.politico.eu/article/vaccine-producers-reject-offers-to-make-more-jabs/>.
- Gaviria M., Kilic B.. 2021. A Network Analysis of COVID-19 mRNA Vaccine Patents. Nature Biotechnology 39(5): 546–48. doi:10.1038/s41587-021-00912-9. [DOI] [PubMed] [Google Scholar]
- Gold E.R. 2021. The Fall of the Innovation Empire and Its Possible Rise through Open Science. Research Policy 50(5): 104226. doi:10.1016/j.respol.2021.104226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herder M. 2021, March 9. Biopharmaceuticals, Financialization & Nationalism in the Age of COVID-19. Healthy Debate. Retrieved January 13, 2022. <https://healthydebate.ca/2021/03/topic/biopharmaceuticals-financialization/>.
- Herder M., Graham J.E., Gold R.. 2020. From Discovery to Delivery: Public Sector Development of the rVSV-ZEBOV Ebola Vaccine. Journal of Law and the Biosciences 7(1): lsz019. doi:10.1093/jlb/lsz019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapczynski A., Chaifetz S., Katz Z., Benkler Y.. 2005. Addressing Global Health Inequities: An Open Licensing Approach for University Innovations. Berkley Technology Law Journal 20: 1032–114. [Google Scholar]
- Krishtel P., Malpani R.. 2021. Suspend Intellectual Property Rights for COVID-19 Vaccines. BMJ 373: n1344. doi:10.1136/bmj.n1344. [DOI] [PubMed] [Google Scholar]
- Leung A.W.Y., Amador C., Wang L.C., Mody U.V., Bally M.B.. 2019. What Drives Innovation: The Canadian Touch on Liposomal Therapeutics. Pharmaceutics 11(3): 124. doi:10.3390/pharmaceutics11030124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moderna Loses Key Patent Challenge. 2020. Nature Biotechnology 38(9): 1009. doi:10.1038/s41587-020-0674-1. [DOI] [PubMed] [Google Scholar]
- Padmanabhan S., Amin T., Sampat B., Cook-Deegan R., Chandrasekharan S.. 2010. Intellectual Property, Technology Transfer and Manufacture of Low-Cost HPV Vaccines in India. Nature Biotechnology 28(7): 671–78. doi:10.1038/nbt0710-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patent Act, R.S.C. 1985, c. P-4. Retrieved January 25, 2022. <https://www.laws-lois.justice.gc.ca/eng/acts/P-4/>.
- UBC University–Industry Liaison Office. n.d. UBC Principles for Access to Technologies Related to COVID-19. The University of British Columbia. Retrieved May 19, 2021. <https://uilo.ubc.ca/technology-transfer/ubc-global-access-principles/ubc-principles-access-technologies-related-covid-19>. [Google Scholar]
- University Global Health Impact Report Card. n.d. Universities Allied for Essential Medicines. Retrieved June 21, 2021. <https://2013.globalhealthgrades.org/>.
- Vardi N. 2021, August 17. COVID's Forgotten Hero: The Untold Story of the Scientist Whose Breakthrough Made the Vaccines Possible. Forbes. Retrieved January 13, 2022. <https://www.forbes.com/sites/nathanvardi/2021/08/17/covids-forgotten-hero-the-untold-story-of-the-scientist-whose-breakthrough-made-the-vaccines-possible/>.
- Wan C., Allen T.M., Cullis P.R.. 2014. Lipid Nanoparticle Delivery Systems for siRNA-Based Therapeutics. Drug Delivery and Translational Research 4(1): 74–83. doi:10.1007/s13346-013-0161-z. [DOI] [PubMed] [Google Scholar]
- Wasan K.M., Thornton S.J., Bell I., Goulding R.E., Gretes M., Gray A.P. et al. 2009. The Global Access Initiative at the University of British Columbia (UBC): Availability of UBC Discoveries and Technologies to the Developing World. Journal of Pharmaceutical Sciences 98(3): 791–94. doi:10.1002/jps.21495. [DOI] [PubMed] [Google Scholar]
- Williams H.L. 2013. Intellectual Property Rights and Innovation: Evidence from the Human Genome. Journal of Political Economy 121(1): 1–27. doi:10.1086/669706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaworski E., Lam K., Jeffs L., Palmer L., Maclachlan I.. 2011. Lipid Formulations for Nucleic Acid Delivery (United States Patent No. US8058069B2). Google Patents. Retrieved January 13, 2022. <https://patents.google.com/patent/US8058069B2/en>. [Google Scholar]
- Yaworski E., Jeffs L.B., Palmer L.R.. 2016. Non-Liposomal Systems for Nucleic Acid Delivery (United States Patent No. US9404127B2). Google Patents. Retrieved January 13, 2022. <https://patents.google.com/patent/US9404127B2/en>. [Google Scholar]
- Zimmermann T.S., Lee A.C.H., Akinc A., Bramlage B., Bumcrot D., Fedoruk M.N. et al. 2006. RNAi-Mediated Gene Silencing in Non-Human Primates. Nature 441(7089): 111–14. doi:10.1038/nature04688. [DOI] [PubMed] [Google Scholar]


