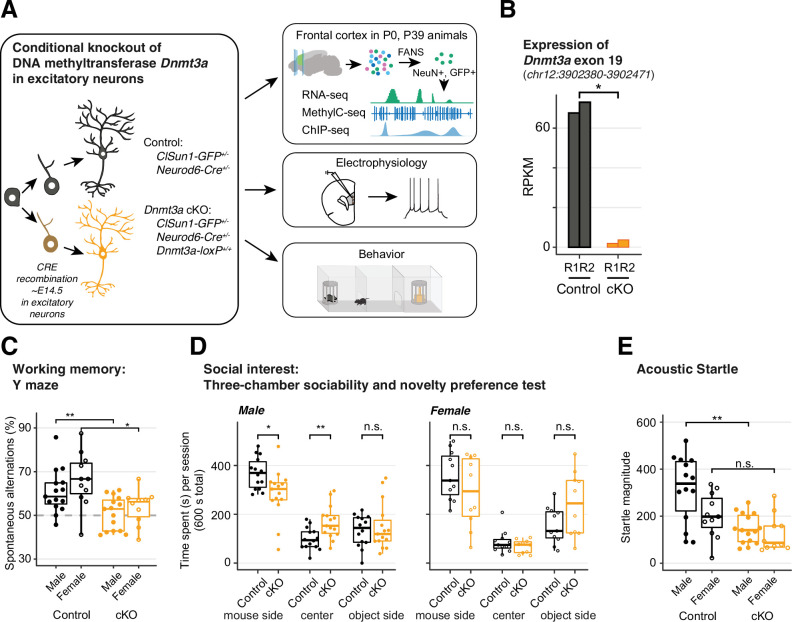
Figure 1. Dnmt3a conditional knockout (cKO) in cortical pyramidal neurons during mid-gestation impaired working memory, social interest, and acoustic startle responses.
(A) An experimental model of the conditional loss of Dnmt3a in excitatory neurons. P0 and P39, postnatal day 0 and 39. FANS, fluorescence-activated nuclei sorting. (B) RNA-seq confirmation of the deletion of Dnmt3a exon 19 in P39 excitatory neurons. RPKM, reads per kilobase per million. R1/2, replicate 1/2. *, t-test p=0.014. (C) Dnmt3a cKO mice made fewer spontaneous alternations in the Y-maze test of working memory (Wilcoxon test, **, p=0.0079; *, p=0.011; n=15 male control, 15 male cKO, 11 female control, 10 female cKO). (D) Male Dnmt3a cKO mice spent less time interacting with an unfamiliar mouse, indicating reduced social interest (Wilcoxon test; *, p=0.01048; **p=0.006833; n=14 male control, 15 male cKO, 11 female control, 10 female cKO). (E) Male Dnmt3a cKO mice had decreased startle response to a 120 dB acoustic pulse (Wilcoxon test, **, p=0.0019; n.s., not significant; n=14 male control, 15 male cKO, 11 female control, 10 female cKO).