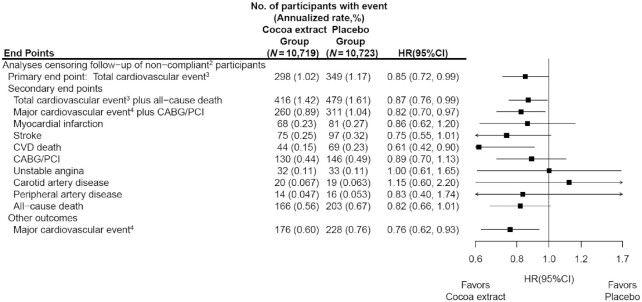
FIGURE 4.
HRs and 95% CIs1 for the primary and secondary CVD outcomes, according to randomized assignment, where follow-up of noncompliant participants was censored. 1Summary statistics were from weighted Cox regression models that stratified baseline hazard functions by multivitamin trial randomization group, age, sex, and recruitment cohort, and used the robust sandwich estimator for variance. Time-dependent weights were calculated as the inverse probability of compliance, where probabilities were estimated from a Cox regression model with baseline hazard functions stratified by age, sex, recruitment source, multivitamin-trial arm and cocoa extract trial arm, and included baseline history of CVD and hypertension status (time-dependent) as covariates. CIs were not adjusted for multiple comparisons. 2A participant's follow-up was censored at the first time they reported having missed >8 d of cocoa extract study pills per month, took personal nonstudy cocoa extract, or did not respond to a semiannual questionnaire. 3Total cardiovascular event was a composite of myocardial infarction, stroke, CVD death, CABG/PCI, unstable angina including hospitalization, carotid artery surgery, and peripheral artery surgery. 4Major cardiovascular event was a composite of myocardial infarction, stroke, and CVD death. CABG/PCI, coronary artery bypass graft and percutaneous coronary intervention; CVD, cardiovascular disease.