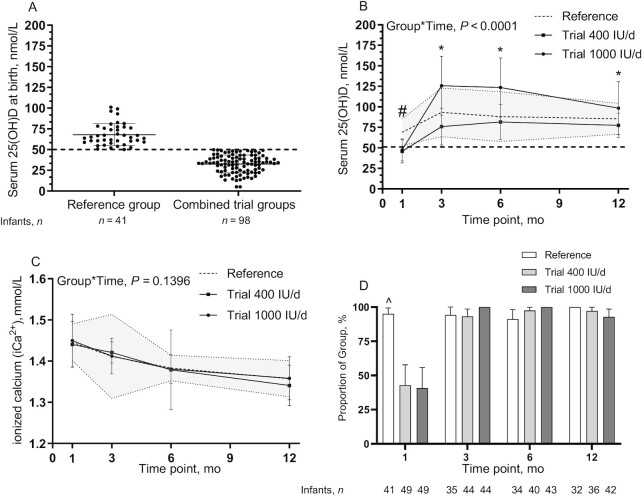
FIGURE 4.
Infant serum 25(OH)D concentrations of the reference group (n = 41) and those in the pool for the trial (n = 98) at birth (24–36 h postpartum) (A), infant serum 25(OH)D concentrations among groups over the first 12 mo of life (baseline, 3, 6, and 12 mo) (B), infant whole-blood ionized calcium among groups (C), and proportions of infants in the reference, trial 400 IU/d, and trial 1000 IU/d groups achieving the cutoff for sufficiency for vitamin D status [25(OH)D ≥50 nmol/L] at each time point (D). (A–C) Data are mean ± SD, (D) data are mean and 95% CIs. Sample sizes are as follows: reference group (baseline, n = 41; 3 mo, n = 35; 6 mo, n = 34; 12 mo, n = 32); 400 IU/d group (baseline, n = 49; 3 mo, n = 44; 6 mo, n = 40; 12 mo, n = 36); and 1000 IU/d group (baseline, n = 49; 3 mo, n = 44; 6 mo, n = 43; 12 mo, n = 42). Continuous data were compared using a mixed-effect model (SAS PROC MIXED) tested for fixed effects of group × time, sex, skin tone, infant actual age at each visit, gravida, parental age at delivery, maternal BMI preconception, and family income. Participant (ID) modeled as a random effect. P values reflect Tukey's post hoc tests with Tukey-Kramer adjustment for multiple comparisons. The shaded area is reference ± SD. #P < 0.05, reference group compared with trial 400 and 1000 IU/d groups; *P < 0.05, trial 1000 IU/d compared with the trial 400 IU/d and reference groups. Categorical data were compared at each time point using chi-square analyses: ^P < 0.05, reference group compared with the trial 400 IU/d and 1000 IU/d groups at baseline only. 25(OH)D, 25-hydroxyvitamin D.