Abstract
Introduction
We investigated the potential association of COVID-19 vaccination with three acute neurological events: Guillain-Barré syndrome (GBS), transverse myelitis and Bell’s palsy.
Methods
With the approval of NHS England we analysed primary care data from >17 million patients in England linked to emergency care, hospital admission and mortality records in the OpenSAFELY platform. Separately for each vaccine brand, we used a self-controlled case series design to estimate the incidence rate ratio for each outcome in the period following vaccination (4–42 days for GBS, 4–28 days for transverse myelitis and Bell’s palsy) compared to a within-person baseline, using conditional Poisson regression.
Results
Among 7,783,441 ChAdOx1 vaccinees, there was an increased rate of GBS (N = 517; incidence rate ratio 2·85; 95% CI2·33–3·47) and Bell’s palsy (N = 5,350; 1·39; 1·27–1·53) following a first dose of ChAdOx1 vaccine, corresponding to 11.0 additional cases of GBS and 17.9 cases of Bell’s palsy per 1 million vaccinees if causal. For GBS this applied to the first, but not the second, dose. There was no clear evidence of an association of ChAdOx1 vaccination with transverse myelitis (N = 199; 1·51; 0·96–2·37). Among 5,729,152 BNT162b2 vaccinees, there was no evidence of any association with GBS (N = 283; 1·09; 0·75–1·57), transverse myelitis (N = 109; 1·62; 0·86–3·03) or Bell’s palsy (N = 3,609; 0·89; 0·76–1·03). Among 255,446 mRNA-1273 vaccine recipients there was no evidence of an association with Bell’s palsy (N = 78; 0·88, 0·32–2·42).
Conclusions
COVID-19 vaccines save lives, but it is important to understand rare adverse events. We observed a short-term increased rate of Guillain-Barré syndrome and Bell’s palsy after first dose of ChAdOx1 vaccine. The absolute risk, assuming a causal effect attributable to vaccination, was low.
Keywords: COVID-19 vaccines, Guillain-Barré syndrome, Bell’s palsy, Transverse myelitis, Self-controlled case series, Vaccine safety
Abbreviations: GBS, Guillain-Barré syndrome; SCCS, self-controlled case series
1. Introduction
Safe vaccination with high uptake is essential to the COVID-19 pandemic response. In England, three COVID-19 vaccines have been widely used: one adenovirus-vectored vaccine ChAdOx1 (Vaxzevria; Oxford AstraZeneca), and two mRNA vaccines, BNT162b2 (Cominarty; Pfizer BioNTech), and mRNA-1273 (Spikevax; Moderna). Each demonstrated a reassuring safety profile in large clinical trials before authorisation [1], [2], [3]. COVID-19 vaccination is highly effective at preventing COVID-19 infection, which can otherwise cause a range of complications, including acute neurological events such as Guillain-Barré syndrome, transverse myelitis and Bell’s palsy [4]. However, post-licensing vaccine safety surveillance of potential adverse events remains essential for vaccine safety and public confidence.
Guillain-Barré syndrome (GBS) is an acute autoimmune polyradiculopathy [5]. Two patients developed GBS in a trial of an adenovirus-vectored vaccine (Ad.26-COV2.S), one of whom received the placebo [6]. British, European and US regulators have issued warnings based on spontaneous reports of GBS following vaccination with adenovirus-vectored vaccines [7], [8], [9]. A large self-controlled case series in England and Scotland reported an association between hospital admission for GBS and first dose of ChAdOx1 vaccine but not BNT162b2 vaccine [4]. Cohort studies in Mexico and Israel have found few cases of GBS following BNT162b2 vaccine [10], [11].
Transverse myelitis is a focal monophasic inflammation of the spinal cord. Trials of ChAdOx1 were paused for safety review following three cases of transverse myelitis (one in the placebo arm) [12]. There have been case reports of transverse myelitis, including longitudinally extensive transverse myelitis, after both COVID-19 infection and vaccination with a range of platforms [13]. The outcome is rare and hypothesis-testing studies to date have been under-powered [4], [13], [14].
Bell’s palsy is an acute, idiopathic unilateral facial paralysis [15]. There was a small imbalance in the number of cases of Bell’s palsy between vaccinated and control groups in trials for mRNA vaccines BNT162b2 and mRNA-1273, although not for the adenovirus-vectored ChAdOx1 vaccine [12], [16], [17]. This has been followed by case reports of Bell’s palsy, predominantly following mRNA platform vaccines [18]. Safety studies have been reassuring for mRNA platform COVID-19 vaccines [4], [14], [18], [19], [20]. However, two UK-based self-controlled case series studies of ChAdOx1 vaccine and Bell’s palsy have produced conflicting results, with one finding no evidence of an association using primary care records [14], and the other finding an increased risk of hospital admission with Bell’s palsy following a first dose of ChAdOx1 vaccine [4]. Hospital admission is not typically required for individuals with Bell’s palsy, and the study described overlap of diagnoses of Bell’s palsy and GBS. Case reports of GBS presenting with facial diplegia (which could be mistaken for Bell’s palsy) following adenovirus-vectored vaccines suggest GBS could drive the finding of increased hospital admission with Bell’s palsy after ChAdOx1 vaccine [21].
We aimed to investigate any association between brand-specific first and second dose of COVID-19 vaccination with each of Guillain-Barré syndrome, transverse myelitis, or Bell’s palsy, using linked primary, emergency care and secondary care electronic health records in England.
2. Material and methods
The pre-specified study protocol is archived at https://github.com/opensafely/Published-Protocols/blob/master/Vaccine%20safety%20protocol_neuro_v1.0.pdf.
2.1. Study design
The study used a self-controlled case series (SCCS) design, a case-only design which compares incidence of events within the same vaccinated individual across different time periods [22].
2.2. Data source
We used data from primary care records managed by the GP software provider TPP, representing approximately 40% of the population in England. Records were linked to Secondary Uses Service (SUS) hospital admission and emergency care data sets and Office for National Statistics mortality data to detect neurological outcomes, National Immunisation Management System (NIMS) data for occupational status, and Second Generation Surveillance System (SGSS) pillar two SARS-CoV-2 test results for prior COVID-19 infection. Data were accessed, linked and analysed through OpenSAFELY-TPP (https://opensafely.org/), a data analytics platform created on behalf of NHS England to address urgent COVID-19 research questions [23].
2.3. Study population
The eligible population comprised all adults (aged 18–105 years) continuously registered at a general practice for at least a year prior to 1 July 2020, to ensure that records represented incident outcomes rather than retrospective recording of past events [24]. Patients with missing age, sex, or postcode, and women with evidence of pregnancy in the nine months before baseline were excluded, as advice on vaccination in pregnancy has evolved during the study period [25].
The study start date was 1 July 2020 to provide a five-month pre-vaccination comparison period before the national vaccination programme began on 8 December 2020. The initial reduction in healthcare attendance for conditions other than COVID-19 infection at the start of the pandemic had considerably recovered for acute physical conditions by July 2020 [26], [27]. The study end date was 7 July 2021, three weeks prior to the latest SUS record availability, to allow completion and recording of most hospital admissions started during the study period [28].
Each analysis included individuals who received a first COVID-19 vaccination dose of the relevant brand and also experienced a new episode of the relevant outcome during the study period, separately for each combination of vaccine brand and outcome.
2.4. Study measures
All codelists and code are available at https://github.com/opensafely/covid-vaccine-safety-research.
2.4.1. Exposures
The primary exposure of interest was brand-specific first dose of COVID-19 vaccination, determined from primary care records and categorised as ChAdOx1, BNT162b2 or mRNA-1273. For secondary analysis, a second dose was defined as the next record of COVID-19 vaccination at least 21 days after the first dose.
2.4.2. Outcomes
Study outcomes were Guillain-Barré syndrome, transverse myelitis, or Bell’s palsy, each considered separately.
For each outcome, we excluded any individuals with the diagnosis of interest recorded during the year prior to 1 July 2020. The first recorded diagnosis of each outcome during follow-up defined the start of a new episode during the study. Any subsequent codes for the outcome during follow-up were considered part of the same episode.
A first diagnosis could be recorded in any of primary care, hospital admission or mortality records. Bell’s palsy was additionally ascertained using emergency care records, as individuals may attend emergency care directly for Bell’s palsy without requiring hospital admission [29]. Emergency care records were not used to ascertain GBS as diagnosis usually requires inpatient investigation and is unlikely to be confirmed within an emergency care attendance [5]. Transverse myelitis diagnoses cannot be recorded in the emergency care dataset [30].
As pre-specified in our protocol, individuals were not eligible for a diagnosis of GBS following a diagnosis of chronic inflammatory demyelinating polyneuropathy (CIDP) recorded ever before or during the study. Similarly, individuals were not eligible for a diagnosis of transverse myelitis following a diagnosis of multiple sclerosis or neuromyelitis optica, since episodes of transverse myelitis symptoms may be differently or under-recorded among patients with these conditions.
2.4.3. Covariates
Confounding factors which do not vary over the study period, such as baseline age and co-morbidities, were automatically controlled for by the SCCS study design. Age was described on 31 March 2021 for consistency with national vaccine eligibility criteria. A history of COVID for stratified analysis was ascertained from a positive COVID-19 PCR test result prior to the vaccination date, obtained from SGSS. Health and social care worker status for sensitivity analysis used self-reported occupation in the NIMS dataset.
2.5. Statistical methods
A separate analysis was conducted for each combination of vaccine brand and outcome.
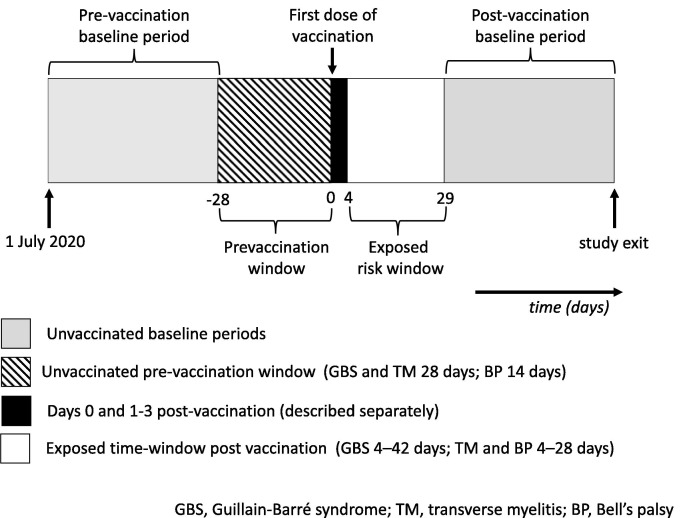
The primary analysis was an unadjusted conditional Poisson regression comparing time periods of interest with baseline time for the same individual to calculate incidence rate ratios. Time windows were defined relative to the individual’s vaccination date (Fig. 1 ). The pre-specified risk window of interest for post-vaccination exposure was 4─42 days after first dose vaccination for GBS and 4─28 days after first dose vaccination for transverse myelitis and Bell’s palsy, with longer risk windows considered in sensitivity analysis, informed by Brighton Collaboration guidance [31], [32], [33], [34]. The risk window was not further subdivided due to the small numbers of cases.
Fig. 1.
Illustration of time-windows for self-controlled case series.
If vaccination is deferred while unwell with neurological symptoms this may violate the assumption of the SCCS design that an outcome event (such as GBS) should not alter the probability of subsequent exposure to vaccination [22], [35]. To allow for this, we defined a separate pre-vaccination window of four weeks for transverse myelitis and Guillain-Barré syndrome, and two weeks for Bell’s palsy. During this pre-vaccination window a lower rate of outcomes is expected, as people experiencing study outcomes are likely to defer vaccination. Defining this period as a separate pre-vaccination window (rather than including it in the baseline time) aims to avoid underestimation of baseline rates. We also defined the day of vaccination as a separate risk window, to exclude opportunistic recording of outcomes on the day of vaccination from the pre-vaccination period (days 0 and 1–3 results not reported due to small numbers, to protect patient confidentiality, but please see Fig. S2 for the distribution of events over time relative to vaccination) [36].
The unexposed baseline time period combined time pre-vaccination (1 July 2020 to the start of the individual’s pre-vaccination window), with time after vaccination (the end of the individual’s post-vaccination risk window until the end of follow-up).
To explore potential changes in ascertainment over time such as under-recording of recent events due to data lags, we explored adjusting for calendar time by week and by fortnight (we report by fortnight as weekly adjustment did not further change results). To investigate any potential interactions with age or previous exposure to SARS-CoV-2 we stratified primary analysis by a history of COVID-19 infection prior to vaccination and by age group.
Data management was performed using Python, with analysis carried out using Stata 16.1 MP.
2.5.1. Secondary analyses
As a secondary analysis, we considered the second dose of vaccination as a separate risk window. In this analysis, day 4 post second dose of vaccination started a separate second-dose post-vaccination risk window, censoring the first-dose risk window if needed. Pre-exposure, day 0 and days 1–3 windows were included for second dose vaccination only if and when the post-vaccination risk window from the first dose had ended.
Another secondary analysis compared first dose of ChAdOx1 vaccine to first dose of BNT162b2 vaccine head-to-head as an active comparator, using a ratio-of-ratios approach. This analysis was limited to vaccinations from 1 January 2020, the period in which both vaccines were available.
2.5.2. Sensitivity analyses
We conducted a number of descriptive and sensitivity analyses to test the study assumptions. We checked for event-dependent censoring of observation time using histograms describing time from outcome to end of follow-up, and used exposure-centred interval plots to assess the suitability of the duration of the pre-vaccination and post-vaccination risk windows.
Pre-specified sensitivity analyses included not removing a pre-exposure window, extending the length of the risk window to 42 days for Bell’s palsy and transverse myelitis and 90 days for GBS, using only post-vaccination follow-up time as the baseline comparator period, and excluding health and social care workers (details in Supplement B).
2.5.3. Post-hoc sensitivity analyses
Adjusting for calendar time slightly increased the incidence rate ratio for transverse myelitis following ChAdOx1 vaccine (Table 1 ). Examining transverse myelitis incidence over time, week 44 was an outlier with approximately double the average number of weekly cases. We excluded week 44 from the ChAdOx1 and transverse myelitis analysis to explore the sensitivity of results to this outlier.
Table 1.
Characteristics of cases in ChAdOx1 vaccine analyses and incidence rate ratios of Guillain-Barré Syndrome, transverse myelitis and Bell’s palsy following ChAdOx1 vaccination.
| Guillain-Barré Syndrome | Transverse myelitis | Bell’s palsy | ||
|---|---|---|---|---|
| Total cases | N | 517 | 199 | 5,350 |
| Age (years) | median [IQR] | 62 [52–72] | 56 [45–65] | 59 [49–71] |
| Female | n (%) | 229 (44·3) | 106 (53·3) | 2,558 (47·8) |
| Analysis | Exposure period | IRR (95% CI) | IRR (95% CI) | IRR (95% CI) |
| First vaccine dose (primary analysis) | ||||
| First vaccine dose, unadjusted (primary analysis) | Baseline | 1 (reference) | 1 (reference) | 1 (reference) |
| Pre-exposure1 | 0·69 (0·45–1·05) | 0·26 (0·10–0·69) | 0·71 (0·60–0·84) | |
| Post-vaccination2 | 2·85 (2·33–3·47) | 1·51 (0·96–2·37) | 1·39 (1·27–1·53) | |
| adjusted for calendar time (fortnight) | Baseline | 1 (reference) | 1 (reference) | 1 (reference) |
| Pre-exposure1 | 0·67 (0·42–1·09) | 0·32 (0·11–0·93) | 0·69 (0·58–0·83) | |
| Post-vaccination2 | 2·46 (1·82–3·32) | 1·96 (1·09–3·52) | 1·31 (1·18–1·46) | |
| Primary analysis, stratified by history of COVID-19 infection | ||||
| History of COVID-19 infection | N (%) | 59 (10·1) | 18 (9·1) | 381 (7·3) |
| Baseline | 1 (reference) | 1 (reference) | 1 (reference) | |
| Post-vaccination2 | 1·15 (0·49–2·72) | 2·51 (0·73–8·67) | 1.11 (0·76–1·63) | |
| No history of COVID-19 infection | Baseline | 1 (reference) | 1 (reference) | 1 (reference) |
| Post-vaccination2 | 3·06 (2·49–3·76) | 1·41 (0·87–2·30) | 1·41 (1·29–1·56) | |
| Primary analysis, stratified by age | ||||
| 18–39 years | N (%) | 35 (6·8) | 26 (153·1) | 561 (10·5) |
| Baseline | 1 (reference) | 1 (reference) | 1 (reference) | |
| Post-vaccination2 | 2·56 (1·15–5·71) | 1·70 (0·51–5·68) | 1·73 (1·33–2·25) | |
| 40–64 years | N (%) | 255 (49·3) | 121 (69·8) | 2,782 (52·0) |
| Baseline | 1 (reference) | 1 (reference) | 1 (reference) | |
| Post-vaccination2 | 3·65 (2·78–4·79) | 1·55 (0·87–2·76) | 1·62 (1·43–1·83) | |
| 65–105 years | N (%) | 227 (43·9) | 52 (26·1) | 2,007 (37·5) |
| Baseline | 1 (reference) | 1 (reference) | 1 (reference) | |
| Post-vaccination2 | 2·12 (1·53–2·93) | 1·32 (0·52–3·33) | 1·01 (0·85–1·20) | |
| First and second doses as separate exposures | ||||
| First and second dose, unadjusted | Baseline | 1 (reference) | 1 (reference) | 1 (reference) |
| D1 Pre-exposure1 | 0·69 (0·45–1·06) | 0·25 (0·09–0·68) | 0·71 (0·60–0·84) | |
| D1 Post-vaccination2 | 2·87 (2·35–3·51) | 1·49 (0·95–2·34) | 1·40 (1·27–1·53) | |
| D2 pre-vaccination3 | 0·80 (0·19–3·37) | 0·57 (0·08–4·27) | 0·60 (0·36–1·00) | |
| D2 post-vaccination4 | 2·13 (0·94–4·83) | N/A | 1·43 (1·08–1·88) | |
| Adjusted for calendar time (fortnight) | Baseline | 1 (reference) | 1 (reference) | 1 (reference) |
| D1 Pre-exposure1 | 0·67 (0·42–1.09) | 0·32 (0·11–0.91) | 0·69 (0·58–0·83) | |
| D1 Post-vaccination2 | 2·46 (1·82–3·33) | 1·89 (1·05–3·39) | 1·31 (1·18–1·46) | |
| D2 pre-vaccination3 | 0·64 (0·15–2·70) | 1·47 (0·06–3·60) | 0·55 (0·33–0·92) | |
| D2 post-vaccination4 | 1·73 (0·75–3·99) | N/A | 1·32 (0·99–1·75) | |
IQR, 1st and 3rd quartile points; IRR, conditional incidence rate ratio; 95% CI, 95% confidence interval; D1, first dose; D2, second dose; N/A, model could not be run due to small number of events.
1. Pre-vaccination window defined as 28 days before vaccination for GBS and transverse myelitis, 14 days for Bell’s palsy.
2. Post-vaccination risk window defined as 4–42 days post vaccination for GBS, 4–28 days for transverse myelitis and Bell’s palsy.
3. Second dose pre-vaccination window started only if and when the first dose post vaccination risk window was completed.
4. Second dose post-vaccination risk window defined as 4–42 days post second-dose vaccination for GBS, 4–28 days for transverse myelitis and Bell’s palsy.
To explore whether the association between first dose of ChAdOx1 vaccine and Bell’s palsy could be explained by misdiagnosis of GBS presenting with facial diplegia, we restricted analyses of Bell’s palsy to cases recorded in primary care, as primary care Bell’s palsy diagnoses have previously been found to have a high positive predictive value.[36].
In the secondary analysis considering the second dose of vaccination as a separate exposure, we added a post hoc sensitivity analysis in case an event after a first dose of vaccine altered the probability of a second dose of vaccine. To do this, we censored the first-dose baseline time at 12 weeks after the first dose (the point at which individuals were routinely eligible for a second dose), so that the baseline time used to calculate risk following the first dose of vaccine would not be affected by different uptake or timing of the second dose of vaccine among people who had already experienced an outcome. We also limited the second-dose baseline time to time after a second dose of vaccination, so that the analysis of risk following the second dose was independent of previous events.
2.5.4. Ethics committee approval
This study was approved by the Health Research Authority (REC reference 20/LO/0651) and by the LSHTM Ethics Board (reference 21863).
3. Results
Among 7,783,441 recipients of ChAdOx1 vaccine, we included 517 adults with a new episode of GBS at any point during the study period (before or after vaccination), 199 with transverse myelitis, and 5,350 with Bell’s palsy (Table 1). Among 5,729,152 recipients of BNT162b2 vaccine, 283 individuals had new episodes of GBS, 109 transverse myelitis, and 3,609 Bell’s palsy. Among 255,446 recipients of mRNA-1273 vaccine, 78 individuals had Bell’s palsy, and there were too few outcomes to investigate GBS or transverse myelitis. There were 36 ChAdOx1 vaccinees and six BNT162b2 vaccinees with a diagnosis of both Bell’s palsy and GBS during the study.
Most GBS and transverse myelitis cases were recorded in hospital records (Table S1). For Bell’s palsy, in the ChAdOx1 analysis 27% of cases were recorded only in primary care, 12% only in emergency care and 27% only in hospital admissions, and the remainder in more than one data source. Among the 38% of Bell’s palsy cases with an emergency care attendance, most (63%) also had a primary care record. The distribution was similar for BNT162b2 vaccine.
3.1. ChAdOx1 vaccination
During the post-vaccination risk window following ChAdOx1 vaccination there was an increased incidence of GBS (incidence rate ratio, IRR 2·85; 95% confidence interval 2·33–3·47) and Bell’s palsy (1·39; 1·27–1·53). The attributable risk was estimated as 11·0 per one million vaccinees for GBS and 17·9 per one million vaccinees for Bell’s palsy.
Both associations were unchanged by adjustment for calendar time and remained among individuals with no known history of COVID. (Table 1). The relative increase in both GBS and Bell’s palsy post-vaccination was higher among individuals aged 40–64 years compared to those over 65 years, although confidence intervals were broad in stratified analysis for GBS. The post-hoc analysis limited to episodes recorded in primary care did not change the association of first dose of ChAdOx1 vaccine with Bell’s palsy (IRR 1·53, 95% CI 1·36–1·72, Table S2).
There was no clear evidence of an increased incidence of transverse myelitis in the primary analysis (unadjusted IRR 1·51; 0·96–2·37). Adjustment for calendar period by fortnight suggested some borderline evidence of increased incidence in the risk window (adjusted IRR 1·96; 1·09–3·52), which remained when removing the outlier week 44 in post-hoc sensitivity analysis (Table S2).
3.2. BNT162b2 vaccination
There was no difference in the incidence for any study outcome during the post-vaccination risk window following BNT162b2 vaccination, and this remained unchanged by adjustment for calendar time and stratification by history of COVID-19 infection and age (Table 2 ).
Table 2.
Characteristics of cases in BNT162b2 vaccine analyses and incidence rate ratios of Guillain-Barré Syndrome, transverse myelitis and Bell’s palsy following BNT162b2 vaccination.
| Guillain-Barré Syndrome | Transverse myelitis | Bell’s palsy | ||
|---|---|---|---|---|
| Total cases | N | 283 | 109 | 3,609 |
| Age (years) | median [IQR] | 70 [53–80] | 54 [40–67] | 62 [40–77] |
| Female | n (%) | 118 (41·7) | 63 (57·8) | 1,898 (52·6) |
| Analysis | Exposure period | IRR (95% CI) | IRR (95% CI) | IRR (95% CI) |
| First vaccine dose (primary analysis) | ||||
| First vaccine dose, unadjusted (primary analysis) | Baseline | 1 (reference) | 1 (reference) | 1 (reference) |
| Pre-exposure1 | 0·60 (0·34–1·04) | 1·16 (0·58–2·30) | 0·72 (0·59–0·87) | |
| Post-vaccination2 | 1·09 (0·75–1·57) | 1·62 (0·86–3·03) | 0·92 (0·80–1·06) | |
| adjusted for calendar time (fortnight) | Baseline | 1 (reference) | 1 (reference) | 1 (reference) |
| Pre-exposure1 | 0·69 (0·36–1·32) | 1·19 (0·55–2·60) | 0·73 (0·59–0·90) | |
| Post-vaccination2 | 1·00 (0·61–1·64) | 1·49 (0·71–3·10) | 0·89 (0·76–1·03) | |
| Primary analysis, stratified by history of COVID-19 infection | ||||
| History of COVID-19 infection | N (%) | 27 (9·5) | ≤5 | 208 (5·8) |
| Baseline | 1 (reference) | 1 (reference) | 1 (reference) | |
| Post-vaccination2 | 0·69 (0·16–2·94) | NR | 0·60 (0·30–1·23) | |
| No history of COVID-19 infection | Baseline | 1 (reference) | 1 (reference) | 1 (reference) |
| Post-vaccination2 | 1·13 (0·77–1·66) | 1·69 (0·90–3·17) | 0·94 (0·82–1·08) | |
| Primary analysis, stratified by age | ||||
| 18–39 years | N (%) | 27 (9·5) | 26 (23·8) | 901 (25·0) |
| Baseline | 1 (reference) | 1 (reference) | 1 (reference) | |
| Post-vaccination2 | 1·29 (0·38–4·37) | 1.15 (0·27–4·86) | 0·63 (0·45–0·89) | |
| 40–64 years | N (%) | 87 (30·7) | 49 (45·0) | 1,048 (29·0) |
| Baseline | 1 (reference) | 1 (reference) | 1 (reference) | |
| Post-vaccination2 | 0·98 (0·49–1·96) | 2·17 (0·91–5·16) | 1·21 (0·98–1·52) | |
| 65–105 years | N (%) | 169 (59·7) | 34 (31·2) | 1,660 (46·0) |
| Baseline | 1 (reference) | 1 (reference) | 1 (reference) | |
| Post-vaccination2 | 1·11 (0·70–1·78) | 1·32 (0·40–4·36) | 0·88 (0·72–1·07) | |
| First and second doses as separate exposures | ||||
| First and second dose, unadjusted | Baseline | 1 (reference) | 1 (reference) | 1 (reference) |
| D1 Pre-exposure1 | 0·61 (0·35–1·06) | 1·12 (0·56–2·23) | 0·71 (0·58–0·87) | |
| D1 Post-vaccination2 | 1·12 (0·77–1·63) | 1·57 (0·84–2·96) | 0·92 (0·81–1·06) | |
| D2 pre-vaccination3 | 0·71 (0·37–1·35) | 0·76 (0·27–2·09) | 0·82 (0·64–1·04) | |
| D2 post-vaccination4 | 1·42 (0·95–2·13) | 0·84 (0·30–2·31) | 1·06 (0·90–1·24) | |
| adjusted for calendar time (fortnight) | Baseline | 1 (reference) | 1 (reference) | 1 (reference) |
| D1 Pre-exposure1 | 0·67 (0·34–1·28) | 1·08 (0·49–2·37) | 0·72 (0·59–0·89) | |
| D1 Post-vaccination2 | 0·93 (0·55–1·56) | 1·34 (0·63–2·82) | 0·88 (0·76–1·02) | |
| D2 pre-vaccination3 | 0·58 (0·28–1·19) | 0·52 (0·17–1·69) | 0·70 (0·54–0·89) | |
| D2 post-vaccination4 | 1·13 (0·67–1·90) | 0·62 (0·20–1·95) | 0·92 (0·78–1·10) | |
IQR, 1st and 3rd quartile points; IRR, conditional incidence rate ratio; 95% CI, 95% confidence interval; D1, first dose; D2, second dose; NR, not reported due to small number suppression.
1. Pre-vaccination window defined as 28 days before vaccination for GBS and transverse myelitis, 14 days for Bell’s palsy.
2. Post-vaccination risk window defined as 4–42 days post vaccination for GBS, 4–28 days for transverse myelitis and Bell’s palsy.
3. Second dose pre-vaccination window started only if and when the first dose post vaccination risk window was completed.
4. Second dose post-vaccination risk window defined as 4–42 days post second-dose vaccination for GBS, 4–28 days for transverse myelitis and Bell’s palsy.
3.3. mRNA-1273 vaccination
There was no evidence of an increased incidence of Bell’s palsy following a first dose of mRNA-1273 vaccination (Table 3 ). Further analysis was not undertaken due to limited power.
Table 3.
Incidence rate ratios of Bell’s palsy relative to first-dose of mRNA-1273 vaccination.
| Outcome | Exposure period | Bell’s palsy |
|---|---|---|
| Total cases | N | 78 |
| Age (years) | median [IQR] | 34 [27–45] |
| Female | n (%) | 36 (46·2) |
| Analysis | Exposure period | IRR (95% CI) |
| Unadjusted (primary analysis) | Baseline | 1 (reference) |
| Pre-exposure1 | 0·66 (0·16–2·69) | |
| Post-vaccination2 | 0·88 (0·32–2·42) | |
| Adjusted for calendar time (fortnight) | Baseline | 1 (reference) |
| Pre-exposure1 | 0·59 (0·13–2·63) | |
| Post-vaccination2 | 0·80 (0·24–2·62) | |
| Post hoc analysis Limited to episodes recorded in primary care (unadjusted) | Baseline | 1 (reference) |
| Pre-exposure1 | 0·47 (0·06–3·36) | |
| Post-vaccination2 | 0·31 (0·04–2·23) |
IRR, conditional incidence rate ratio; 95% CI, 95% confidence interval.
1. Pre-vaccination window defined as 14 days before vaccination.
2. Post-vaccination risk window defined as 4–28 days post-vaccination.
3.4. Secondary analyses
After a second dose of ChAdOx1 vaccine (Table 1) there was some evidence of an increased incidence of Bell’s palsy (IRR 1·43; 95% CI 1·08–1·88), but this was less clear after adjustment for calendar time (1·32; 0·99–1·75) and was no longer present in sensitivity analysis using only post-second dose vaccination time as baseline (0.92; 0.60–1.39) (Table S2). There was no clear evidence of increased incidence of GBS after a second dose of ChAdOx1 vaccine (2·13; 0·94–4·83), and too few events to estimate risk of transverse myelitis.
There was no evidence of increased incidence of any outcome following the second dose of BNT162b2 vaccine (Table 2).
A ratio-of-ratios comparison found that the increase in post-vaccination rate of GBS compared to baseline was twice as high for ChAdOx1 as for BNT162b2 vaccine (ratio of IRRs 2·40, 95% CI 1·57–3·66), and almost 50% higher for Bell’s palsy (1·48, 1·25–1·76) (Table 4 ).
Table 4.
Ratio-of-ratios comparison of ChAdOx1 vaccine relative to BNT162b2 vaccine.
| Outcome | Exposure period | Guillain-Barré Syndrome | Transverse myelitis | Bell’s palsy |
|---|---|---|---|---|
| Ratio of IRRs for ChAdOx1 compared to BNT162b2 (95% CI) | Ratio of IRRs for ChAdOx1 compared to BNT162b2 (95% CI) | Ratio of IRRs for ChAdOx1 compared to BNT162b2 (95% CI) | ||
| Unadjusted | Baseline | 1 (reference) | 1 (reference) | 1 (reference) |
| Pre-exposure1 | 1·06 (0·54–2·13) | 0·21 (0·06–0·69) | 0·95 (0·73–1·24) | |
| Post-vaccination2 | 2·40 (1·57–3·66) | 0·86 (0·40–1·88) | 1·48 (1·25–1·76) | |
| Adjusted for calendar time (fortnight) | Baseline | 1 (reference) | 1 (reference) | 1 (reference) |
| Pre-exposure1 | 0·95 (0·47–1·93) | 0·21 (0·06–0·71) | 0·92 (0·70–1·19) | |
| Post-vaccination2 | 2·22 (1·44–3·41) | 0·85 (0·38–1·88) | 1·43 (1·20–1·71) | |
| With interaction with calendar time (fortnight) | Baseline | 1 (reference) | 1 (reference) | 1 (reference) |
| Pre-exposure1 | 0·94 (0·41–2·16) | 0·24 (0·06–0·90) | 0·92 (0·70–1·22) | |
| Post-vaccination2 | 2·34 (1·29–4·27) | 1·18 (0·45–3·07) | 1·49 (1·23–1·81) |
IRR, conditional incidence rate ratio; 95% CI, 95% confidence interval.
3.5. Sensitivity analyses
There was no evidence of event-dependent censoring shortly after the outcome events (Fig. S1). The exposure-centred interval plots (Fig. S2) suggested the pre-specified pre-vaccination and risk windows were appropriate and there was no evidence of any spikes in outcomes during days 0–3. Consistent with this, a reduced incidence rate ratio was seen during the pre-vaccination window for all outcomes other than transverse myelitis prior to BNT162b2 vaccination (Table 1, Table 2). Extending the post-vaccination risk window to 4–90 days after vaccination slightly attenuated the association of ChAdOx1 with GBS. Otherwise the main analysis results were robust to sensitivity analyses (Tables S2 and S3). All results are available on our github repository: https://github.com/opensafely/covid-vaccine-neuro-research.
4. Discussion
This large self-controlled case series study found a short-term increased incidence of Guillain-Barré syndrome and Bell’s palsy following a first dose of ChAdOx1 vaccine. There was no clear evidence of any association of transverse myelitis with either ChAdOx1 or BNT162b2. There was no evidence of increased incidence of GBS or Bell’s palsy following first or second dose of BNT162b2 vaccination, nor of any increased incidence of Bell’s palsy after mRNA-1273 vaccination.
A previous large self-controlled case series by Patone et al. found an association between first dose of ChAdOx1 vaccine and hospital admission for GBS (IRR 2.90; 95 %CI 2.15–3.92 at 15–21 days after vaccination). [4] Primary care records have previously been found to have good validity for a current episode of GBS [37]. We included GBS recorded in either primary care or hospital admissions and independently replicated the finding. We found a similar relative risk in our risk window of 4–42 days to the most comparable risk window of 1–28 days in Patone et al (IRR 2.04; 95% CI1.60–2.60). Estimates of excess cases in Patone et al. were lower (3.8/million vaccinated) than in our study, which may reflect higher ascertainment of GBS in our study using linked data and including a longer time period which may be less affected by reduced healthcare attendance during the pandemic. We additionally found no evidence of increased risk of GBS after a second dose of ChAdOx1. The relative increase in both GBS and Bell’s palsy post-vaccination was higher among individuals aged 40–64 years compared to those over 65 years, and age-specific estimates should be considered for benefit-risk analyses of vaccination.
Based on clinical trials and case reports, we had hypothesised that Bell’s palsy might be associated with mRNA vaccination but not adenovirus-vectored vaccination. The previous finding by Patone et al. of an association between first-dose ChAdOx1 vaccination and Bell’s palsy hospital admissions was unexpected, and could perhaps have been driven by GBS presenting with facial diplegia [4], [21]. Bell’s palsy is primarily managed in the community and hospital admissions for Bell’s palsy may have been atypical. However, we replicated this finding for Bell’s palsy cases presenting to any of primary care, emergency departments, or requiring hospital admission, and found that the association remained even when restricted to Bell’s palsy recorded in primary care. The association could still be driven by the Facial Diplegia with Paraesthesias variant of GBS, and GBS should be carefully excluded in patients presenting with bilateral facial palsy after ChAdOx1 vaccine, by asking about parasthaesias and examining deep tendon reflexes, which are typically absent in this GBS variant, but not in Bell’s palsy.
The excess risk of GBS appears to apply only to the first dose of ChAdOx1, but not the second dose. For Bell’s palsy, the evidence of an association with second-dose of ChAdOx1 vaccination did not remain in post hoc analysis designed to address potential bias if events after a first vaccination affected the second dose uptake or timing. Together with our finding that there was no evidence of higher risk among people with a previous positive test for COVID-19 infection, this suggests that previous exposure to SARS-CoV-2 infection or vaccination may not increase the future risk of acute neurological complications of vaccination, which is tentatively reassuring for repeated doses of ChAdOx1 vaccine.
We believe this is the first hypothesis-testing study to investigate transverse myelitis following ChAdOx1 or BNT162b2 vaccine. Our results are reassuring, although as transverse myelitis is rare the estimate is imprecise, and a small association cannot be excluded. Adjustment for calendar time slightly increased the incidence rate ratio for transverse myelitis following ChAdOx1. If there is an increased risk of transverse myelitis, it remains too rare an outcome and too low a relative risk for the association to be detected among the general adult population in our study.
Our findings are reassuring for the safety of mRNA vaccines with respect to acute neurological outcomes. We add to the existing evidence on safety of BNT162b2 for GBS and Bell’s palsy [4], [14], [19], [20], and report first analyses of both BNT162b2 vaccine and transverse myelitis, and mRNA-1273 with Bell’s palsy, both reassuring although a small association cannot be ruled out.
Strengths of this study include the large and representative study population with sufficient follow-up to assess the safety of second dose of ChAdOx1 and BNT162b2 vaccine, and to include mRNA-1273 vaccine. We believe these are the first hypothesis-testing studies of transverse myelitis following ChAdOx1 and BNT162b2, which is particularly useful since the high-profile pause of clinical trials for this outcome may influence spontaneous adverse event reporting used in surveillance [12]. The SCCS study design is not susceptible to confounding by fixed individual characteristics, even for characteristics which are unmeasured, although it can be influenced by time-varying confounding which we explored by adjusting for calendar time [22]. We used linked primary and secondary data to enhance ascertainment of the study outcomes, and also emergency care records for Bell’s palsy. We found that Bell’s palsy cases present separately to each of primary care, emergency care and hospital admissions, suggesting that this data linkage is valuable for future research into Bell’s palsy. We conducted multiple sensitivity analyses to explore how robust our findings are to our assumptions and potential sources of bias.
Key limitations include the fact that despite the use of multiple linked data sources, we may have under-ascertained outcomes, particularly if healthcare access was impacted by the COVID-19 pandemic. To minimise potential ascertainment bias, our pre-vaccination comparison period did not include time pre-pandemic, nor the early months of the pandemic in which primary and secondary care attendance were reduced. There was a small overlap of Bell’s palsy and GBS diagnoses and we do not have access to free text in medical notes to investigate potential Bell’s palsy misdiagnosis. Finally, some estimates are imprecise and we cannot exclude a small increase in risk, particularly for the rare outcome of transverse myelitis. However, our findings offer reassurance that the outcome was sufficiently rare and any increased risk sufficiently small that it could not be detected in this large study.
This study investigated the safety of ChAdOx1, BNT162b2 and mRNA-1273 vaccines with respect to acute neurological events in England. Our results support the previous finding of an association of first dose of ChAdOx1 vaccination with GBS and Bell’s palsy. We found that this appears to be specific to the first dose and not the second dose for GBS, and potentially also for Bell’s palsy once the second vaccine dose was considered independently of the first dose. This is tentatively encouraging for repeated doses of ChAdOx1 vaccine. If the associations are causal, the absolute risks attributable to vaccination are low (<1/100,000 for GBS and <1/60,000 for Bell’s palsy). It is possible that the increased incidence of Bell’s palsy reflects presentation of GBS with facial diplegia, which should be carefully excluded among patients with bilateral facial palsy following ChadOx1 vaccination. Our other results were reassuring. The benefits of COVID-19 vaccination are well-established. From the perspective of preventing acute neurological events, increased rates of GBS, transverse myelitis and Bell’s palsy have also been found after COVID-19 infection [14], which COVID-19 vaccines prevent [1], [2], [3]. Our findings should encourage continued confidence in vaccination for policy-makers, vaccinators and the public.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: [JLW, NA and HIM are funded by the NIHR Health Protection Research Unit in Vaccines and Immunisation, a partnership between UK Health Security Agency and London School of Hygiene & Tropical Medicine. JLW and HIM have been occasional invited experts to the Commission on Human Medicines (CHM) COVID-19 Vaccines Safety Surveillance Methodologies Expert Working Group. BS received a grant from the Medical Research Council, via the UKRI/NIHR Global Effort on COVID-19 Research to study neurological disease in relation to COVID-19, and is an unpaid case management consultant to WHO-South-East Asia Region for the COVID-19 pandemic, but vaccination against the infection is not the focus in either case. KD is employed by the Medicines and Healthcare products Regulatory Agency (MHRA) which is an Executive Agency of the Department of Health and Social Care and is the UK Licensing Authority. The MHRA has statutory responsibility to monitor the safety of medicinal products, including vaccines, on the UK market. EW has received payments from AZ for providing training, unrelated to the submitted work. TS was Chair/Co-Chair of the United Kingdom Research and Innovation / National Institute for Health Research COVID-19 Rapid Response and Rolling Funding Initiatives, was an Advisor to the UK COVID-19 Therapeutics Advisory Panel and is a member of the UK Medicines and Healthcare Products Regulatory Agency COVID-19 Vaccines Benefit Risk Expert Working Group. IJD has received unrestricted research grants and holds shares in GlaxoSmithKline (GSK). BG has received research funding from the Laura and John Arnold Foundation, the NHS National Institute for Health Research (NIHR), the NIHR School of Primary Care Research, the NIHR Oxford Biomedical Research Centre, the Mohn-Westlake Foundation, NIHR Applied Research Collaboration Oxford and Thames Valley, the Wellcome Trust, the Good Thinking Foundation, Health Data Research UK (HDRUK), the Health Foundation, and the World Health Organization; he also receives personal income from speaking and writing for lay audiences on the misuse of science.].
Acknowledgments
Acknowledgements
This work uses data provided by patients and collected by the NHS as part of their care and support (http://www.usemydata.org).
We are very grateful for all the support received from the TPP Technical Operations team throughout this work, and for generous assistance from the information governance and database teams at NHS England/NHSX.
Funding
This study was supported by the National Institute for Health Research (NIHR) Health Protection Research Unit (HPRU) in Vaccines and Immunisation and the NIHR HPRU in Emerging and Zoonotic Infections. The OpenSAFELY data science platform is funded by the Wellcome Trust. TPP provided pro bono technical expertise and infrastructure.
JLW, NA and HIM are funded by the National Institute for Health Research (NIHR) Health Protection Research Unit in Vaccines and Immunisation (NIHR200929), a partnership between UK Health Security Agency and LSHTM. AS is employed by LSHTM on a fellowship sponsored by GSK. AT is funded by the UK National Institute for Health Research via academic clinical fellowship scheme. BS is funded by the UK Medical Research Council COVID-Neuro Global programme (MR/V033441/1) and the UK National Institute for Health Research Global Health Research Group on Brain Infections (17/63/110). EW holds grants from MRC. RME is funded by HDR-UK and the MRC. RM holds a Sir Henry Wellcome Fellowship funded by the Wellcome Trust. BMK is also employed by NHS England working on medicines policy and clinical lead for primary care medicines data. KB holds a Wellcome Senior Research Fellowship (220283/Z/20/Z). TS is supported by the National Institute for Health Research (NIHR) Health Protection Research Unit in Emerging and Zoonotic Infections (Grant No. NIHR200907), NIHR Global Health Research Group on Brain Infections (No. 17/63/110), the UK Medical Research Council’s Global Effort on COVID-19 Programme (MR/V03). LS reports grants from Wellcome, MRC, NIHR, UKRI, British Council, GSK, British Heart Foundation, and Diabetes UK outside this work. BG’s work on better use of data in healthcare more broadly is currently funded in part by: the Wellcome Trust, NIHR Oxford Biomedical Research Centre, NIHR Applied Research Collaboration Oxford and Thames Valley, the Mohn-Westlake Foundation; all DataLab staff are supported by BG’s grants on this work.
The views expressed are those of the authors and not necessarily those of the NIHR, NHS England, UK Health Security Agency (UK HSA), Medicines and Healthcare products Regulatory Agency (MHRA) or the Department of Health and Social Care (DHSC). Funders had no role in the study design, collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2022.06.010.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
The pre-specified study protocol is archived at https://github.com/opensafely/Published-Protocols/blob/master/Vaccine%20safety%20protocol_neuro_v1.0.pdf.
All data were linked, stored and analysed securely within the OpenSAFELY platform https://opensafely.org/. Data include pseudonymised data such as coded diagnoses, medications and physiological parameters. No free text data are included. All codelists and code for data management and analysis are shared openly for review and re-use under MIT open license at https://github.com/opensafely/covid-vaccine-safety-research. Detailed pseudonymised patient data is potentially re-identifiable and therefore not shared.
Public Involvement
Public involvement events identified studies of COVID-19 vaccine safety using anonymised patient records as a research priority for the NIHR Health Protection Research Unit in Vaccines and Immunisation. OpenSAFELY have developed a publicly available website https://opensafely.org/ through which they invite any patient or member of the public to make contact regarding the OpenSAFELY project.
References
- 1.Medicines and Healthcare products Regulatory Agency (MHRA). Regulation 174 Information for UK healthcare professionals on COVID-19 Vaccine AstraZeneca 2020 29 December 2020]. <https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/948334/Information_for_UK_healthcare_professionals_on_COVID-19_Vaccine_AstraZeneca.pdf>.
- 2.Medicines and Healthcare products Regulatory Agency (MHRA). Regulation 174 Information for UK healthcare professionals on COVID-19 Vaccine Pfizer BioNTech. 2020 10 December 2020 10 December 2020]. <https://web.archive.org/web/20201207114708/https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/941452/Information_for_healthcare_professionals.pdf>.
- 3.Medicines and Healthcare products Regulatory Agency (MHRA). Summary of Product Characteristics for COVID-19 Vaccine Moderna. 2021 19/04/2021 [cited 2021 30/04/2021]. <https://www.gov.uk/government/publications/regulatory-approval-of-covid-19-vaccine-moderna/information-for-healthcare-professionals-on-covid-19-vaccine-moderna>.
- 4.Patone M., et al. Neurological complications after first dose of COVID-19 vaccines and SARS-CoV-2 infection. Nat Med. 2021 doi: 10.1038/s41591-021-01556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leonhard S.E., Mandarakas M.R., Gondim F.A.A., Bateman K., Ferreira M.L.B., Cornblath D.R., et al. Diagnosis and management of Guillain-Barré syndrome in ten steps. Nat Rev Neurol. 2019;15(11):671–683. doi: 10.1038/s41582-019-0250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marquez Loza A.M., et al. Guillain-barre syndrome in the placebo and active arms of a COVID-19 vaccine clinical trial: temporal associations do not imply causality. Neurology. 2021 doi: 10.1212/WNL.0000000000011881. [DOI] [PubMed] [Google Scholar]
- 7.Rosenblum H.G., et al. Use of COVID-19 vaccines after reports of adverse events among adult recipients of janssen (Johnson & Johnson) and mRNA COVID-19 vaccines (Pfizer-BioNTech and Moderna): update from the advisory committee on immunization practices – United States, July 2021. MMWR Morb Mortal Wkly Rep. 2021;70(32):1094–1099. doi: 10.15585/mmwr.mm7032e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.European Medicines Agency. COVID-19 vaccine safety update: Vaxzevria AstraZeneca AB (8 September 2021). 2021 8 September 2021 [cited 2022 11/1/2022]. <https://www.ema.europa.eu/en/documents/covid-19-vaccine-safety-update/covid-19-vaccine-safety-update-vaxzevria-previously-covid-19-vaccine-astrazeneca-8-september-2021_en.pdf>.
- 9.UK Health Security Agency. Information for healthcare professionals on Guillain-Barré Syndrome (GBS) following COVID-19 vaccination 2021 17 [December 2021 11/1/2022]. <https://www.gov.uk/government/publications/covid-19-vaccination-guillain-barre-syndrome-information-for-healthcare-professionals/information-for-healthcare-professionals-on-guillain-barre-syndrome-gbs-following-covid-19-vaccination>.
- 10.Garcia-Grimshaw M., et al. Guillain-Barre syndrome is infrequent among recipients of the BNT162b2 mRNA COVID-19 vaccine. Clin Immunol. 2021;230:108818. doi: 10.1016/j.clim.2021.108818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shasha D., et al. Real-world safety data for the Pfizer BNT162b2 SARS-CoV-2 vaccine: historical cohort study. Clin Microbiol Infect. 2021 doi: 10.1016/j.cmi.2021.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voysey M., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2020 doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan E., Shrestha A.K., Colantonio M.A., Liberio R.N., Sriwastava S. Acute transverse myelitis following SARS-CoV-2 vaccination: a case report and review of literature. J Neurol. 2022;269(3):1121–1132. doi: 10.1007/s00415-021-10785-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li X, et al. Association between covid-19 vaccination, SARS-CoV-2 infection, and risk of immune mediated neurological events: population based cohort and self-controlled case series analysis. BMJ 2022; 376: e068373. [DOI] [PMC free article] [PubMed]
- 15.Leite A., Andrews N.J., Thomas S.L. Assessing recording delays in general practice records to inform near real-time vaccine safety surveillance using the Clinical Practice Research Datalink (CPRD) Pharmacoepidemiol Drug Saf. 2017;26(4):437–445. doi: 10.1002/pds.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shemer A., Pras E., Hecht I. Peripheral facial nerve palsy following BNT162b2 (COVID-19) vaccination. Isr Med Assoc J. 2021;23(3):143–144. [PubMed] [Google Scholar]
- 19.Shemer A., Pras E., Einan-Lifshitz A., Dubinsky-Pertzov B., Hecht I. Association of COVID-19 vaccination and facial nerve palsy: a case-control study. JAMA Otolaryngol Head Neck Surg. 2021;147(8):739. doi: 10.1001/jamaoto.2021.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McMurry R., Lenehan P., Awasthi S., Silvert E., Puranik A., Pawlowski C., et al. Real-time analysis of a mass vaccination effort confirms the safety of FDA-authorized mRNA COVID-19 vaccines. Med (N Y) 2021;2(8):965–978.e5. doi: 10.1016/j.medj.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonifacio G.B., et al. Bilateral facial weakness with paraesthesia variant of Guillain-Barre syndrome following Vaxzevria COVID-19 vaccine. J Neurol Neurosurg Psychiatry. 2021 doi: 10.1136/jnnp-2021-327027. [DOI] [PubMed] [Google Scholar]
- 22.Petersen I., Douglas I., Whitaker H. Self controlled case series methods: an alternative to standard epidemiological study designs. BMJ. 2016;354:i4515. doi: 10.1136/bmj.i4515. [DOI] [PubMed] [Google Scholar]
- 23.Secretary of State for Health - UK Government. Coronavirus (COVID-19): notification to organisations to share information; 2020 [October 6, 2020]. <https://www.gov.uk/government/publications/coronavirus-covid-19-notification-of-data-controllers-to-share-information>.
- 24.Lewis J.D., Bilker W.B., Weinstein R.B., Strom B.L. The relationship between time since registration and measured incidence rates in the General Practice Research Database. Pharmacoepidemiol Drug Saf. 2005;14(7):443–451. doi: 10.1002/pds.1115. [DOI] [PubMed] [Google Scholar]
- 25.Public Health England. Immunisation against Infectious Disease (the Green Book). Chapter 14a: COVID-19. (Provisional guidance subject to MHRA approval of vaccine supply).
- 26.Mansfield K.E., Mathur R., Tazare J., Henderson A.D., Mulick A.R., Carreira H., et al. Indirect acute effects of the COVID-19 pandemic on physical and mental health in the UK: a population-based study. Lancet Digit Health. 2021;3(4):e217. doi: 10.1016/S2589-7500(21)00017-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walker A.J., et al. Changes in the rate of cardiometabolic and pulmonary events during the COVID-19 pandemic. medRxiv. 2021 2021.02.17.21251812. [Google Scholar]
- 28.NHS Improvement. The long-stays dashboard. 2019 3 July 2019 2 February 2021]. <https://improvement.nhs.uk/resources/long-stays-dashboard/>.
- 29.Fahimi J., Navi B.B., Kamel H. Potential misdiagnoses of Bell's palsy in the emergency department. Ann Emerg Med. 2014;63(4):428–434. doi: 10.1016/j.annemergmed.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Royal College of Emergency Medicine. Emergency care diagnosis simple reference set (foundation metadata concept); 2017 3 April 2017 [cited 2021 28 June 2021]. <https://dd4c.digital.nhs.uk/dd4c/publishedmetadatas/intid/710>.
- 31.Sejvar J.J., Kohl K.S., Gidudu J., Amato A., Bakshi N., Baxter R., et al. Guillain-Barré syndrome and Fisher syndrome: case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2011;29(3):599–612. doi: 10.1016/j.vaccine.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 32.Law, B. and S.P.f.E.v. (SPEAC), SO2- D2.5.2.1 - AESI Case Definition Companion Guide for 1st Tier AESI: Facial nerve palsy. 2021.
- 33.Law B. Safety Platform for Emergency vACcines (SPEAC), SO2-D2.5.2.1 -AESI Case Definition Companion Guidefor 1st Tier AESI: Acute myelitis; 2020.
- 34.Rowhani-Rahbar A., Klein N.P., Dekker C.L., Edwards K.M., Marchant C.D., Vellozzi C., et al. Biologically plausible and evidence-based risk intervals in immunization safety research. Vaccine. 2012;31(1):271–277. doi: 10.1016/j.vaccine.2012.07.024. [DOI] [PubMed] [Google Scholar]
- 35.Whitaker H.J., Ghebremichael-Weldeselassie Y., Douglas I.J., Smeeth L., Farrington C.P. Investigating the assumptions of the self-controlled case series method. Stat Med. 2018;37(4):643–658. doi: 10.1002/sim.7536. [DOI] [PubMed] [Google Scholar]
- 36.Stowe J., Andrews N., Wise L., Miller E. Bell's palsy and parenteral inactivated influenza vaccine. Hum Vaccin. 2006;2(3):110–112. doi: 10.4161/hv.2790. [DOI] [PubMed] [Google Scholar]
- 37.Stowe J., Andrews N., Wise L., Miller E. Investigation of the temporal association of Guillain-Barre syndrome with influenza vaccine and influenzalike illness using the United Kingdom General Practice Research Database. Am J Epidemiol. 2009;169(3):382–388. doi: 10.1093/aje/kwn310. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The pre-specified study protocol is archived at https://github.com/opensafely/Published-Protocols/blob/master/Vaccine%20safety%20protocol_neuro_v1.0.pdf.
All data were linked, stored and analysed securely within the OpenSAFELY platform https://opensafely.org/. Data include pseudonymised data such as coded diagnoses, medications and physiological parameters. No free text data are included. All codelists and code for data management and analysis are shared openly for review and re-use under MIT open license at https://github.com/opensafely/covid-vaccine-safety-research. Detailed pseudonymised patient data is potentially re-identifiable and therefore not shared.
Public Involvement
Public involvement events identified studies of COVID-19 vaccine safety using anonymised patient records as a research priority for the NIHR Health Protection Research Unit in Vaccines and Immunisation. OpenSAFELY have developed a publicly available website https://opensafely.org/ through which they invite any patient or member of the public to make contact regarding the OpenSAFELY project.