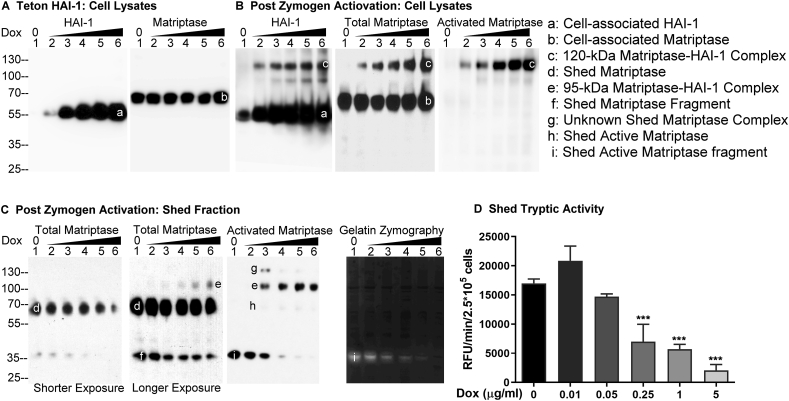
Figure 1.
Increasing HAI-1 expression results in increasing suppression of the shedding of active matriptase. (A) Daudi cells were engineered to express HAI-1 in a doxycycline-regulated manner (Teton HAI-1). The cells were treated with increasing concentrations of doxycycline. Lysates prepared from the same number of cells were then analyzed by Western blot for HAI-1 (left panel) and matriptase (right panel) levels. (B–D) Teton HAI-1 cells were induced to express increasing levels of HAI-1 by treating the cells with increasing concentrations of doxycycline, after which the cells in equal numbers were induced to activate matriptase by acid buffer exposure. The cell lysates (B) and the conditioned buffer (Shed Fraction) (C) were collected and adjusted to the same volumes. An equal volume of the cell lysates (B) and the shed fraction (C) were analyzed by Western blot for HAI-1 species (B), total matriptase (B) and (C), and activated matriptase (B) and (C), and by gelatin zymography for the gelatinolytic activity (C), and by the amidolytic activity assay using a fluorogenic peptide substrate (D). The matriptase and HAI-1 species, including cell-associated HAI-1 (band a), cell-associated matriptase (band b), the 120-kDa matriptase-HAI-1 complex (band c), shed matriptase (band d), shed 95-kDa matriptase-HAI-1 complex (band e), degraded matriptase fragment (band f), as yet unidentified matriptase complex (band g), shed active matriptase (band h), and shed active matriptase fragment (band i) are indicated. Shorter and longer exposures are also indicated. These experiments to study the impact of HAI-1 expression on the shedding of active matriptase were performed at least three times, and representative data are presented. ns: nonsignificant difference; ∗∗∗: P < 0.001.