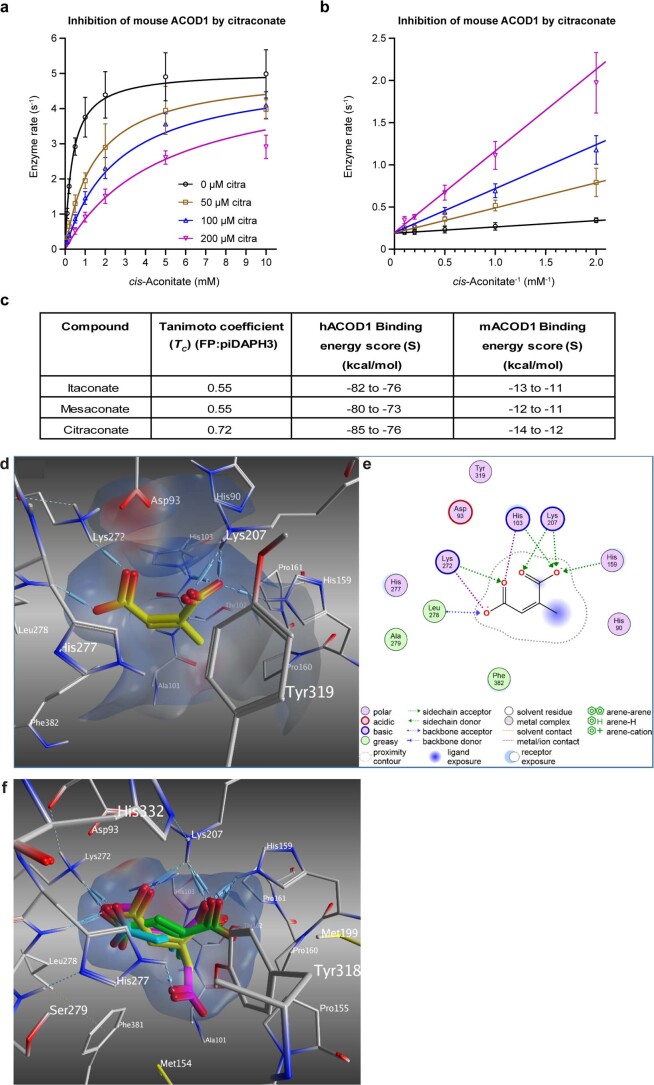
Extended Data Fig. 7. Citraconate is a competitive inhibitor of ACOD1.
a,b, Cell-free assay. Recombinant mACOD1 was incubated with increasing concentrations of substrate (cis-aconitate) and inhibitor (citraconate) and itaconate accumulation was measured by HPLC. n=3 independent assays, means ±SD. Kinetic data pertaining to the values listed in Fig. 3c. The line represents a curve fit to the Michaelis–Menten equation with a competitive inhibitor. b, Lineweaver–Burk plot of the data shown in a. c, Similarity to cis-aconitate (Tanimoto coefficient) and binding energies of the itaconate isomers to human and murine ACOD1. d, Putative binding mode of citraconate (yellow) in the active site of mouse ACOD1 (PDB ID: 6R6T)1. The C1- and C4-carboxyl groups of citraconate bind electrostatically via hydrogen bonds and ion contacts (dashed lines) to the active site residues (His103, His159, Lys207, Lys272, and Leu278). Electrostatic protein surface at the active site: positive (blue), negative (red), neutral (white). e, 2D ligand interactions of citraconate. f, Overlay of putative binding modes of citraconate (yellow), itaconate (cyan), and mesaconate (green) compared to that of the substrate cis-aconitate (magenta) in the active site of human ACOD1 (PDB ID: 6R6U)1. Only citraconate can adopt the same binding mode as cis-aconitate, where the cis-oriented carboxyl groups are fully involved in the interactions with the active-site residues (His159, Lys207, Lys272, and Leu278). In contrast, the carboxyl groups of itaconate and mesaconate interact partially resulting in lower binding affinity.