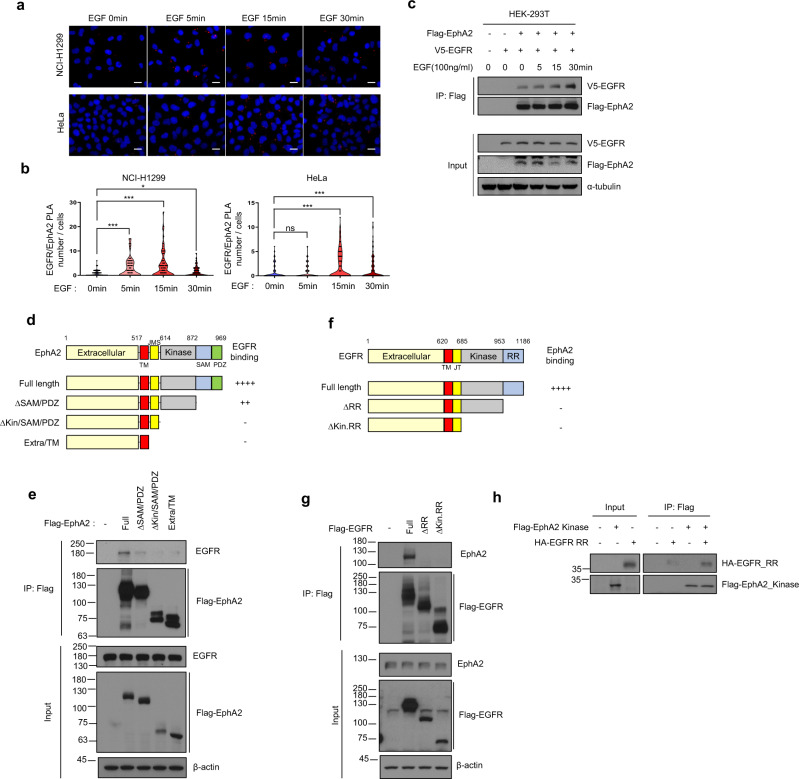
Fig. 2. Ephexin1 mediates interactions between the kinase domain of EphA2 and the RR domain of EGFR.
a Proximity ligation assay (PLA) was used to detect a complex between EphA2 and EGFR in H1299 and HeLa cells that were starved for serum for 16 h and then treated with EGF (100 ng/ml). Localization of EphA2 and EGFR together is shown in red. The cells were counterstained with DAPI (blue) to visualize the nuclei. Scale bar = 20 μm b Quantification of the PLA data shown in (a). c Immunoprecipitation (IP) with an anti-Flag antibody of protein extracts from HEK-293T cells co-transfected with Flag-tagged EphA2 and V5-tagged EGFR, that were treated with EGF (100 ng/ml) after a 16 h serum starvation. Western blot analysis was performed with indicated antibodies. d Schematic representation of wild type EphA2 and a series of deletion mutants. A summary of the degree to which each interacts with EGFR is shown to the right. e Lysates from HEK293T cells transfected with Flag-tagged wild type EphA2 or deletion mutants were immunoprecipitated with anti-Flag antibody and subjected to western blot analysis with the indicated antibodies. f Schematic representation of wild type EGFR and a series of deletion mutants. A summary of the degree to which each interacts with EphA2 is shown to the right. g Lysates from HEK293T cells transfected with Flag-tagged wild type EGFR or deletion mutants was immunoprecipitated with anti-Flag antibody and subjected to western blot analysis with indicated antibodies. h Pulldown assay to measure the binding of Flag-tagged EphA2_kinase domain with HA-tagged EGFR_RR domain. Immunoprecipitation was carried out using the anti-Flag antibody.