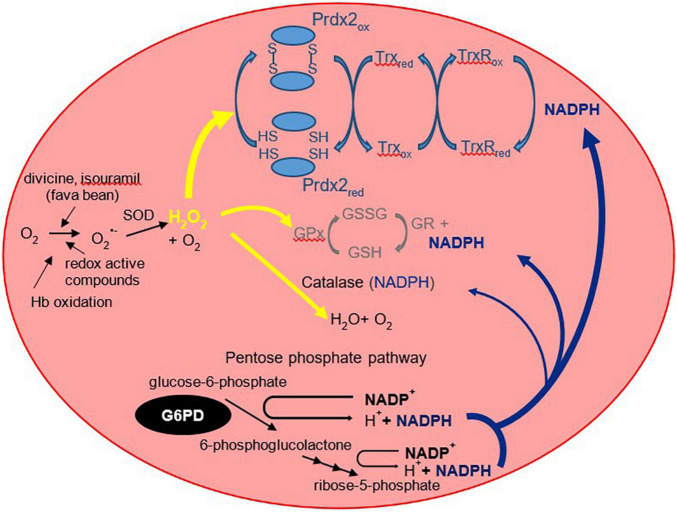
FIGURE 1.
The three RBC antioxidant systems that are compromised in G6PD deficiency. The more familiar glutathione peroxidase/glutathione reductase (GPx/GR) and antioxidant mechanisms are shown in gray (middle) and the more recently recognized peroxiredoxin 2 system in light blue (top). Prdx2 is a thiol protein that is oxidized to an interchain disulfide then recycled predominantly by thioredoxin (Trx) and thioredoxin reductase [TrxR; (102)]. Prdx2, the third most abundant RBC protein, is present at a much higher concentration than glutathione peroxidase or catalase. It is highly reactive with peroxides and is favored to consume most of the intracellular H2O2 (17). NADPH is required as a reducing substrate for both GR and TrxR, and it protects catalase against inactivation. It is produced via the pentose phosphate pathway of which the first step is catalyzed by G6PD. By restricting the supply of NADPH, G6PD deficiency compromises the ability of all three antioxidant systems to detoxify hydrogen peroxide. O2–, superoxide; H2O2, hydrogen peroxide; GSH, reduced glutathione; GSSG, glutathione disulfide; SOD, superoxide dismutase. The “red” and “ox” subscripts refer, respectively, to the reduced and oxidized forms of the proteins.