Abstract
Background
Cognitive impairment is a core feature of disorders on the schizophrenia-bipolar spectrum, i.e., schizophrenia, bipolar disorder, and schizoaffective disorder. Brain-derived neurotrophic factor (BDNF) has been proposed to be a biomarker of cognitive impairment in these disorders as it plays a critical role in neuroplasticity and proposed to mediate some of the psychotropic effects of medication. However, despite numerous studies investigating the association between circulating BDNF and these disorders, no solid conclusions have been drawn regarding its involvement in cognitive impairment.
Objectives
The current systematic review and meta-analysis aims to examine blood BDNF levels and cognitive dysfunction in patients on the schizophrenia-bipolar spectrum as well as to evaluate whether circulating BDNF measurements can act as a biomarker for cognitive dysfunction.
Methods
Studies were identified by searching Embase and Medline databases for English language articles published in peer-reviewed journals between 2000 January and 2021 June according to the PRISMA guidelines. A total of 815 articles were identified of which 32 met the inclusion criteria for the systematic review – reporting on comparisons between blood BDNF levels and cognitive functions of schizophrenia or bipolar disorder patients versus healthy controls (no studies involving schizoaffective patients were specifically obtained for the time being). Twenty-four of these studies (19 with schizophrenia and 5 with bipolar disorder patients) were eligible to be included in the meta-analysis.
Results
Our findings indicated that circulating BDNF levels were significantly reduced in patients experiencing an acute episode of schizophrenia or bipolar disorder compared to healthy controls. Cognitive function was also found to be significantly worse in patients, however, correlations between BDNF levels and cognitive impairment were not always detected. Interventions, especially pharmacotherapy seemed to improve certain aspects of cognition and increase circulating BDNF levels.
Conclusion
Circulating BDNF alone does not seem to be a valid biomarker of cognitive dysfunction in patients with disorders on the schizophrenia-bipolar spectrum, owing to several confounding factors. Changes of the circulating levels of BDNF should be evaluated in a wider context of other stress-, immune-, and inflammatory-related factors.
Keywords: schizophrenia, schizoaffective disorder, bipolar disorder, BDNF, cognition, biomarker
Introduction
Schizophrenia is a serious psychiatric disorder characterized by considerable distortions of thinking and perception driven by three core symptom domains; positive symptoms, negative symptoms, and cognitive dysfunction (1). Bipolar disorder is also a major psychiatric condition, but it is recognized by the alternation of mood episodes and behavioral activation (1). The prevalence of both disorders is around 1% of the general population (2, 3). An intermediate phenotype between schizophrenia and bipolar disorder is schizoaffective disorder, which is characterized by the concurrent occurrence of an equal admixture of both schizophrenic and major affective disorder symptoms cross-sectionally and/or longitudinally (1). Together, the three disorders can be referred to as schizophrenia-bipolar spectrum disorders.
Cognitive dysfunction, defined broadly as the inability to properly process information, has been well established to be a core feature of these disorders (4, 5). In bipolar disorder, cognitive impairment usually manifests in specific cognitive domains such as attention, verbal memory, or executive functioning with greater severity throughout the acute manic-depressive episodes compared to the euthymic states (6–8). In contrast, the same cognitive deficits in schizophrenia tend to be stable across time without considerable improvements between psychotic episodes (9, 10). Deficits in cognition have also been described in schizoaffective disorder and at a greater extent than in patients with bipolar disorder (11). Importantly, cognitive impairment has been proposed to be a crucial factor in achieving improved functioning and quality of life in these patient groups (12–14). However, since currently there are no effective treatments for cognitive impairment in the schizophrenia-bipolar spectrum, it remains a major unmet clinical need (15). Thus, investigating potential biomarkers of cognitive dysfunction is not only crucial for understanding the pathophysiology of disorders on the schizophrenia-bipolar spectrum, but also for the development of potential treatments and interventions (16, 17). One of the candidates for such biomarker is brain-derived neurotrophic factor (BDNF).
Brain-derived neurotrophic factor is a member of the nerve growth factor family, which functions include enhancing the growth and maintenance of various neuronal systems, ensuring neuronal plasticity, modulating neurotransmitter activity, and contributing to learning and memory throughout life (18–21). It facilitates neuronal plasticity via the stimulation of dendritic growth, the formation of synapses as well as neurogenesis in brain areas related to memory such as the hippocampus (21, 22). Activation of BDNF release from axons is influenced negatively by several factors including inflammation, stress as well as age (21). Since BDNF can be readily measured in blood, several studies have associated its peripheral concentrations with central functions and neuropathology. For instance, circulating BDNF levels have been associated with hippocampal volume and spatial memory in older adults, with lower levels of BDNF correlating with smaller volume of the hippocampus and worse performance on neurocognitive tests (23). Furthermore, in terms of stress, animal studies found that social isolation in mice resulted in decreased BDNF levels in several brain areas including the prefrontal cortex, hippocampus, and hypothalamus (24). In view of these results and that it can cross the blood–brain barrier, BDNF has gained considerable attention as a possible biomarker for neurocognitive processes in several psychiatric and neuropsychiatric disorders (25) such as Alzheimer’s disease (26), autism spectrum disorder (27), or disorders on the schizophrenia-bipolar spectrum (28).
Several studies measured the concentrations of BDNF in the blood and examined its relationship with cognitive symptoms in patients on the schizophrenia-bipolar spectrum compared to healthy controls in order to better understand the role of BDNF. The findings of such studies however were quite mixed; a meta-analysis by Ahmed et al. did not observe significant connection between cognitive impairment and BDNF levels in the blood based on five schizophrenia studies (29). Another systematic review and meta-analysis involving 21 studies with schizophrenia patients reported a positive correlation between cognitive impairment and reduced blood BDNF levels, especially in chronic samples (30). In the case of bipolar disorder, meta-analyses have consistently reported reduced BDNF levels in manic and depressive episodes compared to healthy controls (31, 32), but the connection between BDNF levels and cognition in these reviews were not examined.
The present systematic review and meta-analysis aims to update the existing literature regarding circulating BDNF levels and cognitive functioning in the schizophrenia-bipolar spectrum in comparison to healthy controls, in order to conclusively demonstrate whether blood BDNF can act as a biomarker of cognitive dysfunction.
Methods
Search Strategy
Studies for the systematic review were identified by searching Embase and Medline databases for English language articles published in peer-reviewed journals between 1 January 2000 and 1 June 2021 according to the PRISMA guidelines (33) with search terms “(schizo* OR bipolar) AND (BDNF* OR ‘Brain Derived Neurotrophic Factor’) AND (‘Neurocognit*’ OR Cognit*).” Searches by hand and via the reference section of published reports and previous review papers were also conducted in order to identify additional relevant studies.
Inclusion and Exclusion Criteria
The inclusion criteria for studies were the following: (1) original research conducted with human subjects; (2) involved patients with diagnosis on the schizophrenia-bipolar spectrum; (3) included at least one cognitive assessment; (4) reported enzyme-linked immunosorbent assay (ELISA) measurement of BDNF levels in blood serum or plasma; (5) included healthy controls. Papers were excluded if they examined only genetic BDNF data or baseline blood BDNF levels were not adequately reported. Studies that did not report BDNF levels or cognitive scores for the total patient sample were excluded from the meta-analyses.
Statistical Analyses
Means, standard deviations (SDs), and effect sizes were calculated in Microsoft Excel. The effect size was calculated for differences between baseline BDNF levels of schizophrenia patients and healthy controls or bipolar patients and healthy controls in ng/ml using mean, SD, and sample sizes. In case of cognitive scores, the effect size was based on the differences between baseline Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) scores of schizophrenia patients and healthy controls using mean, SD, and sample sizes. As effect size measure, Hedge’s g was computed since the studies included in the meta-analyses had relatively small sample sizes and Hedge’s g is less biased in case when variance equality assumptions are not met.
The meta-analyses were performed using the “meta” package in R studio, with standardized mean difference used as effect size measurement. While Z statistic was calculated to determine the significance of the effect size, Q statistic was computed to provide an estimation of the degree of homogeneity of the effect sizes of the different studies. The degree of inconsistency was signalized with the I2 metric (I2 > 75% indicating large heterogeneity, >50% moderate heterogeneity, and <50% low heterogeneity). To present the effect sizes of individual studies, a forest plot was created.
Due to the heterogeneity of studies, separate analyses for schizophrenia and bipolar disorder patients were performed with three random-effects meta-analyses. The first analysis examined the difference in circulating BDNF levels between schizophrenia patients and healthy controls, the second examined the difference in circulating BDNF levels between bipolar disorder patients and healthy controls, and the third looked at the difference in cognitive functions measured by RBANS between schizophrenia patients and healthy controls. Data was scarce to conduct an analysis for the difference between bipolar disorder patients and healthy controls in terms of cognitive functions. Similarly, conducting a meta-analysis of correlation coefficients was impossible due to the scarcity and heterogeneity of data. In these cases, qualitative analyses were performed.
Results
Search Results
A summary of article selection for the systematic review is presented in the PRISMA chart (Figure 1). A total of 815 articles were identified and 69 were determined as potentially eligible to be included in the review based on the titles and abstracts. After evaluating the articles fully, only 32 met the inclusion criteria as in several articles there were no control group present (21 studies) or the reporting on BDNF levels (6 studies) or cognitive functions (9 studies) was inadequate. The majority of studies (26 studies) were conducted with schizophrenia patients; 16 of them were with either first-episode patients (FEP) (34–41) or patients with chronic schizophrenia (CH) (42–48). In the bipolar studies there were patients in euthymic state (49–52) and in depressive (53) and manic (50) episodes involved. No studies involving schizoaffective patients were obtained which is a relevant shortcoming. Altogether, 4,754 schizophrenia and 476 bipolar disorder patients were compared to 3,526 healthy controls in the systematic review. In terms of level of evidence, most of the studies were level B according to the rating system by Siwek et al., meaning that the design of the studies were of lower quality, mostly case-control studies, and there were only a few randomized controlled trials (level A) (54).
FIGURE 1.
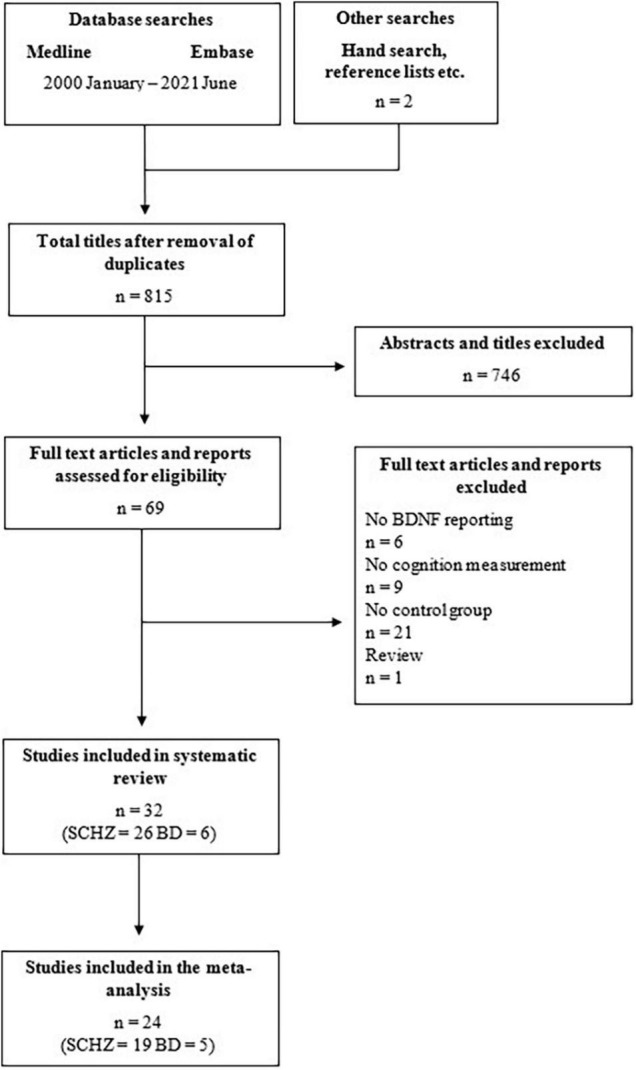
PRISMA flowchart demonstrating the search strategy that was utilized for the systematic review and meta-analysis.
Studies where BDNF levels or RBANS scores were not reported for the total patient population were excluded from the meta-analyses. The first analysis that compared baseline BDNF levels between schizophrenia patients and healthy controls included 19 studies. The second analysis that looked at baseline BDNF levels of bipolar disorder patients versus healthy controls included five studies (49, 51–53, 55). The third and final analysis examined the differences of baseline RBANS scores between schizophrenia patients and healthy controls also included 5 studies (34, 48, 56–58).
Blood Brain-Derived Neurotrophic Factor Levels
Circulating BDNF concentrations were measured either in the plasma (9 studies) or in the serum (23 studies). Statistically significant difference between patients and healthy controls was detected in 25 studies (21 with schizophrenia and 4 with bipolar disorder patients) as summarized in Table 1. Importantly, 24 of these studies reported decreased blood BDNF levels in patients compared to controls; only one study by Asevedo et al. found higher BDNF levels in schizophrenia patients than in controls (59). Out of the seven studies where no significant difference was reported, two were conducted with euthymic bipolar disorder patients (49, 52), while the rest was with schizophrenia patients (37, 46, 60–62). The other two studies examining euthymic bipolar patients found reduced BDNF levels compared to healthy controls, nonetheless, it was highlighted that the levels were still higher than what was found in manic bipolar patients (50, 51).
TABLE 1.
Summary of studies.
| Study | Diagnosis | Patient N | Control N | BDNF type | BDNF in patients versus controls | BDNF ES (95% CI) | Cognition measures | Cognition in patients versus controls | BDNF-cognition relationship | Level of evidence (54) |
| Asevedo et al. (59) | SZ | 30 | 27 | Plasma | ↑ | 1.04 (0.52; 1.57) |
Verbal learning, verbal fluency, working memory, set shifting, inhibition, executive function tests | Deficits in verbal learning | BDNF levels positively correlated with semantic generation tasks | B |
|
| ||||||||||
| Carlino et al. (42) | CH-SZ | 40 | 40 | Serum | ↓ | – | Processing speed, attention, executive function, working memory tests | Significantly poorer neurocognitive performance | Serum truncated-BDNF abundance predicted for high cognitive deficits | B |
|
| ||||||||||
| Chang et al. (55) | BD-II | 228 | 135 | Plasma | ↓ | −0.23 (−0.44; −0.01) |
WMS | Significantly lower scores on 5 subtests of WMS | BDNF more likely to be associated with clinical characteristics than with memory | B |
|
| ||||||||||
| Chou et al. (49) |
EU-BD-I | 23 | 33 | Plasma | NS | −0.02 (−0.55; 0.51) |
Attention, memory, executive function tests | Cognitive deficits present | Deficits in cognition not significantly correlated with BDNF except two items from tests | B |
|
| ||||||||||
| Dell’Osso et al. (53) | D-BD-I | 16 | 15 | Plasma | ↓ | −1.71 (−2.55; −0.87) |
Cognitive disturbances factor score form HRSD | NA | BDNF levels may be related to severity of depression and retardation symptoms | B |
|
| ||||||||||
| Dias et al. (52) | EU-BD-I | 65 | 50 | Serum | NS | 0.19 (−0.18; 0.56) |
Attention and mental control, perceptual-motor skills, executive functions, verbal fluency and abstraction, visuospatial attention, memory tests | Significantly worse results on 11 out of the 16 neurocognitive tests | Significant positive association between serum BDNF levels and a test of verbal fluency in both BD patients and controls | B |
|
| ||||||||||
| Dong et al. (64) | SZ | 818 | 467 | Serum | ↓ | CLZ, MA: −0.85 (−1.02; −0.67) CLZ, FE: −0.13 (−0.40; 0.15) RISP, MA: −0.86 (−1.08; −0.64) RISP, FE: −0.55 (−0.86; −0.24) TYP, MA: −0.99 (−1.19; −0.78) TYP, FE: −0.09 (−0.47; 0−29) |
RBANS | Significantly lower scores | Association between BDNF and cognitive performance in only male patients and female patients taking typical antipsychotics | B |
|
| ||||||||||
| Hori et al. (46) | CH-SZ | 86 | 51 | Serum | NS | −0.32 (−0.67; 0.03) | IGT | Significantly lower scores in IGT | Negative correlation between BDNF levels and mean net scores on the trials in the final two blocks | B |
|
| ||||||||||
| Hori et al. (60) | SZ | 146 | 51 | Serum | NS | −0.24 (−0.56; 0.08) | BACS | NA | Negative correlations between serum BDNF levels and scores for verbal memory, attention and processing speed | B |
|
| ||||||||||
| Li et al. (70) | SZ | 472 | 225 | Serum | ↓ | −1.56 (−1,72; −1.40) | RBANS | Significantly lower RBANS total score | Serum BDNF independently positively correlated with attention and immediate memory | B |
|
| ||||||||||
| Man et al. (34) | FEP-SZ | 80 | 80 | Serum | ↓ | −1.22 (−1.56; −0.88) | RBANS | Significantly lower cognitive performance on the RBANS total and four of its five subscale scores | No significant correlation between BDNF and any index or total scores of RBANS | B |
|
| ||||||||||
| Mora et al. (50) | EU-BD and MA-BD | 84 (52 EU; 32 MA) | 49 | Serum | ↓ | EU: −0.50 (−0.89; −0.10) MA: −0.89 (−1.35; −0.44) |
Executive function, selective attention, inhibition, processing speed, cognitive flexibility, sustained attention, perseverative behavior, verbal learning, recall, recognition, visual memory tests | Worse performance in executive functioning, inhibition, processing speed, verbal and visual memory | BDNF levels associated with executive functioning and verbal memory, together with other demographic variables | B |
|
| ||||||||||
| Niitsu et al. (61) | SZ | 63 | 52 | Serum | NS | 0.17 (0,20; 0.54) |
WAIS-R, VFT, WCST, TMT, Stroop test, DSDT | Significantly worse performance on all tests | Serum BDNF levels related to the impairment of verbal working memory in patients | B |
|
| ||||||||||
| Penadés et al. (62) | SZ | 70 | 15 | Serum | NS | 0.25 (−0.31; 0.81) | Global cognition, working memory, processing speed, verbal memory, non-verbal memory, executive function tests | Significantly worse performance on most tests | No significant correlation between serum BDNF level and cognition | A |
|
| ||||||||||
| Qu et al. (35) | DN-FEP-SZ | 256 | 177 | Serum | ↓ | M: −1.07 (−1.28; −0.86) F: −0.85 (−1.09; −0.62) |
RBANS | Cognitive function decreased | No association between BDNF and cognitive function | B |
|
| ||||||||||
| de Azua et al. (36) | FEP-SZ | 45 | 45 | Plasma | ↓ | −0.78 (−1.20; −0.35) | Learning ability, immediate and delayed memory, abstract thinking, and processing speed tests | Cognitive performance of patients significantly worse | Plasma BDNF levels at 6 months after first hospitalization positively associated with several cognitive domains | B |
|
| ||||||||||
| Rybakowski et al. (51) | EU-BD-I | 60 | 60 | Plasma | ↓ | −0.43 (−0.80; −0.07) | CANTAB | Lithium-treated patients had poorer results on all domains of neuropsychological tests | Performance on neuropsychological tests and plasma BDNF levels in excellent lithium responders is different compared to patients lacking the optimal effect of lithium but not different compared to matched healthy controls | B |
|
| ||||||||||
| Tang et al. (82) | SZ | 109 | 40 | Serum | ↓ | DS: −2.44 (−2.86; −2.02) NDS: −2.25 (−2.66; −1.84) |
Processing speed, attention, executive function, working memory tests | Significantly worse performance | No correlations between BDNF levels and the cognitive tests in SZ and HC groups | B |
|
| ||||||||||
| Theleritis et al. (37) | FEP-SZ | 87 | 152 | Plasma | NS | 0.26 (−0.01; 0−52) | IQ, verbal memory and learning, visual memory, executive function, working memory, attention, concentration, processing speed, verbal fluency tests | NA | No association between BDNF plasma levels and cognitive functions | B |
|
| ||||||||||
| Vinogradov et al. (43) | CH-SZ | 56 | 15 | Serum | ↓ | −0.59 (−1.16; −0.03) | MCCB | Decrement in cognitive functioning (∼ 1 SD below the normal mean) | No significant association between change in BDNF and change in global cognition | A |
|
| ||||||||||
| Wei et al. (44) | CH-SZ | 189 | 60 | Serum | ↓ | −1.00 (−1.30; −0.70) | Executive function test | Executive function impaired | BDNF may be a useful biomarker for executive dysfunction | B |
|
| ||||||||||
| Wu et al. (45) | CH-SZ | 83 | 52 | Serum | ↓ | TD: −1.00 (−1.43; −0.57) WTD: −0.65 (−1.05; −0.25) |
RBANS | Significantly lower scores in almost all subscales | No significant associations between BDNF and RBANS total score or any cognitive index | B |
|
| ||||||||||
| Wu et al. (38) | DN-FEP-SZ | 354 | 152 | Serum | ↓ | −1.08 (−1.27; −0.89) | RBANS | Extensive cognitive impairment | No significant association between BDNF levels and RBANS total score or its index scores | B |
|
| ||||||||||
| Xiao et al. (63) | DN-FEP-SZ | 58 | 55 | Serum | ↓ | −0.99 (−1.39; −0.65) | Verbal fluency, attention and processing speed, attention distribution, working memory, motor speed, and executive function tests | Significantly worse on nearly all neurocognitive tests | BDNF levels positively correlated with the animal subscale of the VFT and negatively correlated with TMT-part B scores | B |
|
| ||||||||||
| Xiu et al. (39) | SZ | 232 | 60 | Serum | ↓ | −0.81 (−1.10; −0.53) | Executive function tests | Significantly lower scores | Lower BDNF levels were correlated with executive dysfunction | B |
|
| ||||||||||
| Xiu et al. (56) | DN-FEP-SZ | 327 | 391 | Serum | ↓ | −0.88 (−1.04; −0.73) | RBANS | Significantly lower scores | No relationship between BDNF and cognitive impairments | B |
|
| ||||||||||
| Yang et al. (40) | FEP-SZ, CH-SZ | 65 34 FEP, 31 CH |
35 | Plasma | ↓ | FEP: −0.44 (−0.91; 0.04) CH: −0.62 (−1.11; −0.13) |
MCCB | Index scores remarkably lower | Low BDNF levels were associated with cognitive impairments | B |
|
| ||||||||||
| Zhang et al. (69) | SZ | 575 | 405 | Serum | ↓ | Val/Val: −0.77 (−1.02; −0.52) Val/Met: −0.82 (−1.00; −0.65) Met/Met: −0.88 (−1.15; −0.60) |
RBANS | Significantly lower in cognitive scores in nearly all subscales | Higher serum BDNF levels were associated with better cognitive function | B |
|
| ||||||||||
| Zhang et al. (48) | CH-SZ | 251 | 206 | Serum | ↓ | −0.93 (−1.13; −0.74) | RBANS | Significantly lower scores | BDNF positively associated with immediate memory | B |
|
| ||||||||||
| Zhang et al. (47) | CH-SZ | 248 | 188 | Serum | ↓ | −0.91 (−1.10; −0.72) | RBANS | Worse performance on most of the cognitive tasks | BDNF positively associated with immediate memory in female patients | B |
|
| ||||||||||
| Zhang et al. (57) | SZ | 108 | 47 | Serum | ↓ | −1.70 (−2,05; −1.36) | RBANS | Significantly lower scores | Metabolic adverse effects of olanzapine may aggravate cognitive dysfunction in patients with schizophrenia through an interaction between BDNF | B |
|
| ||||||||||
| Zhang et al. (58) | AC-SZ | 68 | 47 | Plasma | ↓ | −0.52 (−0.89; −0.14) | RBANS | Decreased compared to controls | Increase in plasma levels of BDNF significantly correlated with the change in the RBANS total scores | B |
AC, acute; BACS, Brief Assessment of Cognition in Schizophrenia; BD, bipolar disorder; BDNF, brain-derived neurotrophic factor; CANTAB, Cambridge Neuropsychological Test Automated Battery; CH, chronic; CLZ, clozapine; D, depressed; DN, drug-naïve; DS, deficit schizophrenia; DSDT, Digit Span Distraction Test; ES, effect size; EU, euthymic; F, female; FEP, first episode; HRSD, Hamilton Rating Scale for Depression; IGT, Iowa Gambling Task; MA, manic; M, male; MCCB, MATRICS Consensus Cognitive Battery; NDS, non-deficit schizophrenia; NA, not available, NS, not significant; RBANS, Repeatable Battery for the Assessment of Neuropsychological; RISP, risperidone; SZ, schizophrenia; TD, tardive dyskinesia; TMT, Trail Making Test; TYP, typical; VFT, Verbal Fluency Test; WAIS-R, Wechsler Adult Intelligence Scale Revised; WCST, Wisconsin Card Sorting Test; WMS, Wechsler Memory Scale; WTD, without tardive dyskinesia.
When analyzing the effect sizes, large effect size (Hedges’ g of 0.8 or larger) was detected in 15 studies (1 study with bipolar and 14 with schizophrenia patients), medium (Hedges’ g of 0.5 to 0.8) in 5 studies, and small (Hedges’ g under 0.5) in 11 studies (Table 1). Large effect sizes were predominantly acquired in studies with first episode schizophrenia (34, 35, 38, 41, 63). In contrast, small effect sizes were more likely to be seen in studies involving bipolar patients (4 out of 6 studies) (49, 51, 52, 55). The smallest effect sizes were associated with patients with CH and/or patients receiving antipsychotic medication monotherapy (40, 46, 58, 60, 61). Importantly, female patients also seemed to have BDNF levels closer to normal compared to males (52, 64).
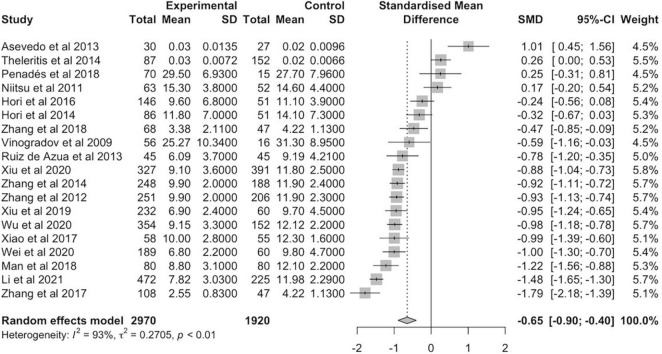
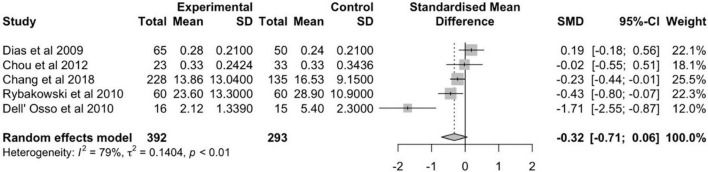
The meta-analysis of 19 schizophrenia studies was conducted with a total of 2,970 patients versus 1,920 healthy controls (Figure 2). The random effects estimate showed a moderate reduction of BDNF levels in schizophrenia patients compared to healthy controls (g = −0.65, 95% CI: −0.90 to −0.40). The level of heterogeneity was high (I2 = 93%, p < 0.01). The meta-analysis of the 5 bipolar disorder studies involved a total of 392 patients and 293 controls (Figure 3). In case of bipolar disorder patients, the random effects model reported a small reduction of BDNF levels in bipolar disorder patients in contrast to health controls (g = −0.32, 95% CI: −0.71 to 0.06) with slightly lower heterogeneity (I2 = 79%, p < 0.01).
FIGURE 2.
Forest plot of standardized mean difference (SMD) in BDNF levels found in blood of patients with schizophrenia and healthy controls.
FIGURE 3.
Forest plot of standardized mean difference (SMD) in BDNF levels found in blood of patients with bipolar disorder and healthy controls.
Cognitive Dysfunction
The assessment of cognitive functions varied within the reviewed literature. Most commonly (12 studies) the RBANS (65) was applied, whereas 7 studies used other validated scales such as the MATRICS™ Consensus Cognitive Battery (MCCB) (40, 43, 66), the Cambridge Neuropsychological Test Automated Battery (CANTAB) (51, 67) or the Brief Assessment of Cognition in Schizophrenia (BACS) (60, 68). The RBANS is a brief test that evaluates five indexes: immediate memory, visuospatial/constructional, language, attention, and delayed memory (65). In contrast, the BACS measures cognition functions via verbal memory, verbal fluency, working memory, motor speed, attention, and executive functioning (68), while the MCCB evaluates peed of processing, attention/vigilance, working memory, verbal learning, visual learning, reasoning and problem solving, and social cognition (66). The rest of the studies chose a combination of individual assessments that measured specific cognitive domains such as executive functioning, inhibition, or different aspects of memory (e.g., verbal or visual memory) via tests such as the Wisconsin Card Sorting Test (WCST) or specific parts of the Wechsler Adult Intelligence Scale (WAIS) (44, 50, 59).
Importantly, almost all studies (29 out of 32) reported significant difference between patients and controls, with patients exhibiting deficits in several cognitive domains. Concerning schizophrenia studies, differences between first episode and chronic patients were detected; higher scores were found in the chronic population compared to the first-episode population on the RBANS, with mean scores of 70.3 and 66.0, respectively (Table 2). Differences between males and females were also prevalent; female patients, especially in chronic populations, had lower cognitive impairment than male patients (35, 47, 64).
TABLE 2.
Baseline RBANS scores in first episode and chronic schizophrenia patients.
| Study | Schizophrenia patients |
Healthy controls |
Effect size | 95% confidence interval | ||
| Subject (sex) | Mean RBANS score (SD) | Subject (sex) | Mean RBANS score (SD) | |||
| First-episode schizophrenia | ||||||
| Man et al. (34) | 80 | 64.0 (12.9) | 80 | 79.0 (12.3) | −1.19 | −1.50, −0.88 |
| Qu et al. (35) |
160 (M) | 66.4 (14.5) | 208 | 82.6 (13.1) | −1.16 | −1.39, −0.94 |
| 118 (F) | 67.0 (17.6) | 181 | 80.5 (15.3) | −0.82 | −1.06, −0.58 | |
| Xiu et al. (56) | 256 | 66.6 (15.9) | 180 | 80.2 (15.0) | −0.88 | −1.07, −0.69 |
| Summary, means | 685 | 66.0 (15.2) | 860 | 81.6 (14.5) | −1.01 | −1.25, −1.77 |
| Chronic schizophrenia | ||||||
| Dong et al. (64) (CLZ) | 357 (M) | 64.9 (14.7) | 193 (M) | 80.2 (15.0) | −1.02 | −1.20, −0.83 |
| 63 (F) | 72.6 (17.4) | 274 (F) | 80.0 (15.3) | −0.46 | −0.74, −0.19 | |
| Dong et al. (64) (RIS) | 135 (M) | 64.2 (16.0) | 193 (M) | 80.2 (15.0) | −1.02 | −1−26, −0.79 |
| 48 (F) | 73.2 (14.3) | 274 (F) | 80.0 (15.3) | −0.44 | −0.75, −0.13 | |
| Dong et al. (64) (TYP) | 184 (M) | 64.6 (13.9) | 193 (M) | 80.2 (15.0) | −1.06 | −1.28, −0.85 |
| 31 (F) | 78.1 (18.4) | 467 (F) | 80.0 (15.3) | −0.12 | −0.48, 0.24 | |
| Wu et al. (45) (WTD) | 35 | 63.9 (9.1) | 52 | 82.4 (12.5) | −0.74 | −1.14, −0.33 |
| 48 | 73.4 (11.6) | 52 | 82.4 (12.5) | −1.62 | −2.11, −1.13 | |
| Zhang et al. (48) | 251 | 71.7 (16.4) | 206 | 76.9 (16.0) | −0.33 | −0.51, −0.14 |
| Zhang et al. (47) | 216 (M) | 71.1 (15.2) | 72 (M) | 79.6 (13.1) | −0.57 | −0.84, −0.30 |
| 63 (F) | 75.1 (17.1) | 90 (F) | 76.9 (14.8) | −0.11 | −0.43, 0.21 | |
| Summary, means | 1,431 | 78.1 (14.9) | 2,066 | 79.9 (13.9) | −0.68 | −1.25, −0.77 |
CLZ, clozapine; F, female; M, male; RISP, risperidone; SD, standard deviation; TYP, typical; WTD, without tardive dyskinesia.
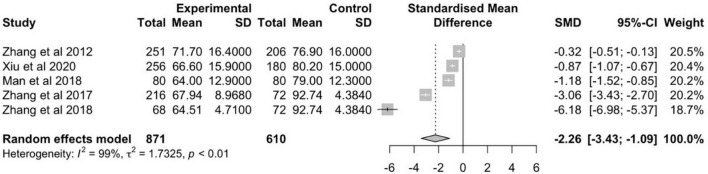
The meta-analysis of 5 schizophrenia studies was conducted with a total of 871 patients versus 610 healthy controls (Figure 4). The random effects estimate showed a large reduction of RBANS scores in schizophrenia patients compared to healthy controls (g = −2.26, 95% CI: −3.43 to −1.09). The level of heterogeneity was high (I2 = 99%, p < 0.01).
FIGURE 4.
Forest plot of standardized mean difference (SMD) in total mean RBANS scores measured in patients with schizophrenia and healthy controls.
In case of bipolar disorder, patients in all states (manic, depressive or euthymic) were found to perform worse than controls on the different cognitive tests (49–52). Interestingly, significant difference between manic compared to euthymic patients was found in only one domain (verbal memory) in a study by Mora et al. (50). Nonetheless, the scores of euthymic patients were still lower than that of healthy controls (50). Similar results were acquired in patients treated with lithium, where poorer results on all cognitive domains compared to controls were reported (51). Importantly, the study also highlighted that excellent lithium responders had numerically better results than non-excellent responders (51). Due to heterogeneity of cognition measurements no meta-analysis could be conducted in patients with bipolar disorder.
Correlation Between Blood Brain-Derived Neurotrophic Factor Levels and Cognitive Dysfunction
Correlations between circulating BDNF levels and cognitive functions in patients were calculated in 26 studies, out of which 16 studies reported Pearson’s correlation coefficients, 5 Spearman’s correlation coefficients and 5 partial correlation coefficients. Again, most of the studies correlated cognitive functions to BDNF serum levels, and only a few to plasma levels. In terms of cognitive functions, total scores, index scores, or individual test scores were included in the correlation analyses. All in all, 19 studies found significant correlations between circulating BDNF levels; correlation coefficients and p-values are shown in Table 3.
TABLE 3.
Correlations between BDNF levels and cognition.
| Study | Diagnosis | BDNF type | Cognition measurement | Correlation measure | Correlation coefficient |
| Asevedo et al. (59) | SZ | Serum | Semantic generation task | Spearman’s correlation | 0.38* |
| Letter memory task | −0.45* | ||||
|
| |||||
| Carlino et al. (42) | CH-SZ | Serum | TMT Part B | Partial correlation | 0.55*** (low truncated BDNF) |
| Digit symbol coding | 0.36* (low truncated BDNF) | ||||
| Digit span forward | 0.36* (low truncated BDNF) | ||||
|
| |||||
| Chou et al. (49) | EU-BD | Plasma | Sounds RT (divided attention) | Partial correlation | Data missing* |
| Faces 2 true positive (faces memory) | Data missing** | ||||
|
| |||||
| Dias et al. (52) | EU-BD | Serum | Test of verbal fluency (COWAT) | Pearson’s correlation | 0.26* |
|
| |||||
| Dong et al. (64) | SZ | Serum | RBANS total score | Pearson’s correlation | 0.18** (CLZ, M) |
| 0.39** (RISP, M) | |||||
| 0.30** (TYP, M) | |||||
| 0.55* (TYP, F) | |||||
|
| |||||
| Hori et al. (46) | CH-SZ | Serum | Card block 61–80 (IGT) | Pearson’s correlation | 0.23* |
| Card block 81–100 (IGT) | 0.27* | ||||
|
| |||||
| Hori et al. (60) | SZ | Serum | Verbal memory (BACS) | Pearson’s and Spearman’s correlation | 0.19* |
| Attention and processing speed (BACS) | 0.16* | ||||
|
| |||||
| Li et al. (70) | SZ | Serum | RBANS total score | Pearson’s correlation | 0.33*** (without T2DM) |
|
| |||||
| Niitsu et al. (61) | SZ | Serum | Information subscale (WAIS-R) | Spearman’s correlation | 0.29* |
|
| |||||
| Wei et al. (44) | CH-SZ | Serum | VFT total score | Partial correlation | 0.33* |
|
| |||||
| Wu et al. (45) | CH-SZ | Serum | RBANS total score | Pearson’s correlation | −0.38* (TD) |
| Immediate memory index (RBANS) | 0.32* (WTD) −0.36* (TD) |
||||
| Delayed memory index (RBANS) | −0.38* (TD) | ||||
|
| |||||
| Wu et al. (38) | DN-FEP-SZ | Serum | Delayed memory index (RBANS) | Pearson’s correlation | −0.26* (high−BDNF) |
|
| |||||
| Xiao et al. (41) | DN-FEP-SZ | Serum | TMT Part B | Spearman’s correlation | −0.40** |
| VFT-animals | 0.27* | ||||
|
| |||||
| Xiu et al. (39) | SZ | Serum | VFT total score | Partial correlation | 0.30* |
| WCST sub-score | −0.27* | ||||
|
| |||||
| Yang et al. (40) | SZ | Plasma | Learning and memory (MCCB) | Pearson’s correlation | 0.28* |
|
| |||||
| Zhang et al. (69) | SZ | Serum | RBANS total score | Pearson’s correlation | 0.21* |
|
| |||||
| Zhang et al. (48) | CH-SZ | Serum | Immediate memory index (RBANS) | Pearson’s correlation | 0.23*** |
|
| |||||
| Zhang et al. (47) | CH-SZ | Serum | RBANS total score | Pearson’s correlation | 0.34** (F) |
| Immediate memory index (RBANS) | 0.51*** (F) | ||||
|
| |||||
| Zhang et al. (58) | AC-SZ | Plasma | RBANS total score | Spearman’s correlation | 0.28* |
| Attention index (RBANS) | 0.27* | ||||
p-Value: *<0.05, **<0.01, ***<0.001.
AC, acute; BACS, Brief Assessment of Cognition in Schizophrenia; BD, bipolar disorder; BDNF, brain-derived neurotrophic factor; CH, chronic; CLZ, clozapine; COWAT, Controlled Oral Word Association Test; DN, drug naïve; F, female; FEP, first episode; EU, euthymic; IGT, Iowa Gambling Task; M, male; MCCB, MATRICS Consensus Cognitive Battery; RBANS, Repeatable Battery for the Assessment of Neuropsychological Status; RISP, risperidone; SZ, schizophrenia; TD, tardive dyskinesia; TMT, Trail Making Test; TYP, typical; VFT, Verbal Fluency Test; WAIS-R, Wechsler Adult Intelligence Scale Revised; WCST, Wisconsin Card Sorting Test; WTD, without tardive dyskinesia.
Most studies reported negligible (r < 0.3) (38–40, 46, 48, 52, 58, 60, 61, 63, 64, 69) or low (0.3 < r < 0.49) (39–42, 44, 45, 59, 64, 70) positive correlations between circulating BDNF levels and cognitive functions and only three studies found moderate (0.5 < r < 0.7) correlations (42, 47, 64). For instance, Dong et al. reported moderate positive correlation between baseline serum BDNF level and RBANS total score but only in female patients taking typical antipsychotic medications (r = 0.55, p < 0.05) (64). In contrast, correlations between BDNF serum levels and RBANS total scores were low in male patients taking typical antipsychotic medications (r = 0.30; p < 0.01) or risperidone (r = 0.39; p < 0.01) (64). Similarly, Zhang et al. found moderate positive correlation between BDNF serum levels and immediate memory index score from RBANS but only in chronic female schizophrenia patients (47). Finally, Carlino et al. found moderate positive correlation between low truncated-BDNF expression and performance on Trail Making Test Part B (r = 0.55; p < 0.001) in CH patients (42).
In general, significant correlations between circulating BDNF levels and cognitive assessments were more prevalent in the CH population, with 6 out of 7 studies reporting statistically significant correlation coefficients (42, 44–48). The specific domains associated with circulating BDNF levels were immediate (45, 47, 69), delayed (45) and working memory (42), decision making (46), speed of processing (42), executive (42), and verbal functioning (44). Interestingly, Wu et al. found different correlation directions depending on whether patients had tardive dyskinesia or not; serum BDNF levels of patients with TD correlated negatively with RBANS total score, and immediate and delayed memory indexes (45).
In case of (drug naïve) first episode patients, non-significant correlations between circulating BDNF levels and cognitive functioning were detected in the majority of studies (34, 35, 56). Only two studies by Wu et al. and Xiao et al. found significant correlations; Wu et al. reported negative correlation between delayed memory index from RBANs and serum BDNF levels (r = −0.26; p < 0.05), however only in patients with high baseline BDNF levels (38), Xiao et al. found negative correlation between serum BDNF levels and executive functioning (r = −0.40; p < 0.01) and positive correlation with verbal function (r = 0.27; p < 0.05) (41).
The rest of the studies examining schizophrenia detected significant correlations between circulating BDNF levels and semantic generation task (59), and verbal memory, attention and processing speed (60), verbal and executive functioning (39), and RBANS total score (58, 69, 70). Interestingly, correlations were different for schizophrenia patients with and without type 2 diabetes mellitus in a study by Li et al.; serum BDNF levels correlated with total RBANS score in non-diabetic schizophrenia patients only (r = 0.33; p < 0.001) (70).
Finally, in bipolar patients, circulating BDNF levels were reported to significantly correlate with specific domains of cognition in 2 out of the 3 studies that calculated coefficients, namely divided attention (p < 0.05) (49), faces memory (p < 0.01) (49), and verbal fluency (r = 0.26; p < 0.05) (52).
Changes in Brain-Derived Neurotrophic Factor Levels and Cognitive Dysfunction After Treatment
Altogether, 4 of the 32 reviewed studies examined the changes in BDNF levels and cognitive functions before and after treatment, all with schizophrenia patients. Two studies focused on the effects of pharmacotherapy (38, 58) and two studies on the impact of non-pharmacological interventions such as cognitive remediation (62) and computerized auditory training (43) as summarized in Table 4. In case of the latter, 56 schizophrenia outpatients were randomized to 10 weeks of computerized auditory training or control condition (computer game) and were compared to 16 matched healthy controls. According to the results, statistically significant change was detected in global cognition as well as BDNF levels in response to the training (43). By week 10, the BDNF levels in patients were comparable to that of healthy controls; at baseline these were significantly lower than of the healthy controls (43). Nonetheless, no significant association was found between the changes in BDNF levels and the cognitive measurements (43). The other study that examined the effects of non-pharmacological treatment on BDNF levels and cognitive impairment randomized 70 patients to either cognitive remediation therapy (CRT) or social skills training (control group) for 4 weeks (62). Although some improvements in cognition were detected, the authors could not report any significant changes in serum BDNF levels in response to the CRT (62). Moreover, the association between cognitive improvements and BDNF levels were also non-significant (62). Both RCTs evaluated cognition via the MCCB (43, 62).
TABLE 4.
Brain-derived neurotrophic factor levels and cognition scores at baseline and after treatment.
| Study | Patient (N) | Treatment | BDNF, mean (SD) |
Cognition measure | Cognition, mean (SD) |
||
| Before | After | Before | After | ||||
| Penadés et al. (62) | 35 | 4-month cognitive remediation | 26.1 (7.4) | 27.9 (9.1) | Global cognition | 43.26 (4.62) | 48.48 (4.32) |
| Working memory | 47.95 (9.91) | 50.35 (8.84) | |||||
| Processing speed | 43.15 (7.21) | 48.82 (6.28) | |||||
| Verbal memory | 37.99 (7.86) | 44.75 (7.71) | |||||
| Non-verbal memory | 44.24 (8.35) | 47.90 (6.12) | |||||
| Executive function | 40.88 (7.94) | 49.11 (6.55) | |||||
| Quality of life EQ-5D | 4.79 (1.12) | 6.74 (1.12) | |||||
|
| |||||||
| Vinogradov et al. (43) | 29 | 50-h computerized auditory training | 25.3 (10.3) | 32.2 (15.1) | Global cognition | – | – |
| Speed of processing | |||||||
| Verbal working memory | |||||||
| Verbal learning | |||||||
| Verbal memory | |||||||
| Problem solving | |||||||
| Non-verbal working memory | |||||||
| Visual learning | |||||||
| Visual memory | |||||||
| Social cognition | |||||||
|
| |||||||
| Wu et al. (38) | 190 | 12-week risperidone monotherapy | 9.1 (3.3) | 10.8 (6.3) | RBANS | – | – |
|
| |||||||
| Zhang et al. (58) | 68 | 12-week olanzapine monotherapy | 3.4 (2.1) | 4.7 (1.7) | RBANS | 322.57 (23.55) | 339.34 (43.51) |
N, number; RBANS, Repeatable Battery for the Assessment of Neuropsychological Status; SD, standard deviation.
In terms of pharmacological therapies, Zhang et al. designed a 12-week open-label, prospective observational trial to examine the effects of olanzapine on BDNF and cognitive functioning and thus to evaluate if BDNF can act as a biomarker for cognition (58). At baseline, the 95 patients exhibited significantly worse cognitive performance as measured by RBANS and lower plasma BDNF levels than the 72 controls (58). In response to olanzapine treatment, significant improvements in immediate memory, attention, and total RBANS score as well as increased plasma BDNF levels compared to baseline were detected (58). Importantly, the increase in BDNF plasma levels showed correlation with the change in the RBANS scores (58). Based on the results, the authors concluded that plasma BDNF levels might be a potential biomarker for cognitive functioning in patients with acute schizophrenia (58).
Similarly, Wu et al. conducted a 12-week, flexible-dose, prospective, observational trial in 354 drug-naïve FEP with schizophrenia (38). The aim was to evaluate the impact of risperidone treatment on serum BDNF levels and cognitive functioning measured by RBANS (38). According to the results, poorer cognitive functioning and lower serum BDNF levels were detected at baseline in patients compared to 152 controls (38). In response to treatment, significant improvement in memory, delayed memory and RBANS total score as well as slight increase in BDNF levels was found (38). Interestingly, when separating patients to low-BDNF and high-BDNF baseline groups, different responses to antipsychotic medication were acquired (38). Those in the low-BDNF group had increased, while those in the high-BDNF group had decreased plasma levels after risperidone treatment (38). In addition, correlations between lower BDNF levels and delayed memory were also detected, but only in patients who had higher baseline BDNF levels (38).
Discussion
To our knowledge this is the first systematic review and meta-analysis that examined circulating BDNF levels and cognitive dysfunction in patients on the schizophrenia-bipolar spectrum. The aim of the paper was threefold: to update the existing literature regarding the differences between patients and healthy controls in blood BDNF levels and cognitive functioning, to compare patients with schizophrenia, bipolar disorder, and schizoaffective disorder in terms of circulating BDNF levels and cognitive dysfunction, and to understand the relationship between BDNF and cognition in these patient populations. The relevance of the results is discussed below.
The results confirmed that there is a moderate reduction in patients with schizophrenia and small reduction in patients with bipolar disorder in serum or plasma BDNF levels compared to healthy controls. The results are in line with previous meta-analyses that also found moderate quality evidence of reduced blood BDNF levels in these patient groups (16, 71–73). Similarly to the results of a comparative meta-analysis by Fernandes et al., the present study also agrees that the decrease in circulating BDNF levels compared to healthy controls is greater in acute patients than in those in chronic or euthymic states (73). Differences in blood BDNF levels also seem to depend on several other factors including sex, age, or medication, which was again shown by previous research as well (74, 75).
In contrast to circulating BDNF levels, cognitive impairment was found to be pronounced in all states and stages of the disorders, confirming that indeed cognitive deficits are a core feature of the schizophrenia-bipolar spectrum. Interestingly however, better scores on different cognition assessments were reported in patients with CH compared to patients in first episode. In terms of the relationship between circulating BDNF levels and cognitive functioning, significant but negligible correlations were found in more than one third of the reviewed studies. Differences between patient groups were also prevalent in this aspect of the analysis as well; significant correlations were more likely to be found in chronic patients compared to first episode patients, and in female patients compared to males.
All in all, circulating BDNF levels alone do not seem to be a valid biomarker of cognitive dysfunction in patients on the schizophrenia-bipolar spectrum. Although BDNF has been repeatedly found to be reduced in patients compared to healthy controls, the correlations between BDNF and cognition are weak. This is especially true for drug naïve first episode patients who have high levels of cognitive dysfunction and low levels of blood BDNF, yet the two are not correlated. Indeed, the relationship between cognition and BDNF is more pronounced in patients with CH, suggesting that factors such as age or state of disorder might be mediating this relationship. This has been proposed by previous reviews as well; although the meta-analysis by Bora et al. found correlation between cognitive symptoms and BDNF levels, they also concluded that the relationship between the two might be rather indirect (30). In addition, Fernandes et al. came to similar conclusions too, suggesting that reduced BDNF levels might be connected to the suppressive effects of stress (73).
If putting these results into context, it is likely that the reason why most reduction in BDNF levels was detected in first-episode, drug naïve patients is due to that fact that these patients experience the highest levels of stress. As the stress levels are lower in chronic, medicated, and euthymic patients, the BDNF levels are less influenced by it and hence correlations between cognitive symptoms are more prevalent. The fact that most correlations in this patient population were found in executive functioning, immediate memory, and processing speed – neurocognitive functions all mediated by the hippocampus and prefrontal cortex – further supports this notion.
Finally, it deserves attention from clinical point of view that blood BDNF levels and cognitive symptoms were found to improve after certain therapies and antipsychotic medications. In case of pharmacotherapy, the improvement in cognitive functioning and circulating BDNF levels were even correlated. Some experimental results suggest that D3 receptors may also play a role in influencing BDNF levels and cognitive improvement (76–79). Thus, further research needs to investigate whether novel medications targeting D3 receptors have different effects on BDNF levels in patients on the schizophrenia-bipolar spectrum and how these potential changes in BDNF levels would relate to cognitive impairment.
Limitations
The main limitation of this systematic review is the heterogeneity of the studies; large differences in sample sizes, patient populations, BDNF measurements (plasma or serum) and cognitive scales were prevalent. Due to this heterogeneity the relationship between BDNF levels and cognitive dysfunction could not be quantified, as neither the Hedges–Olkin nor the Schmidt–Hunter method is suitable for a small number of heterogeneous studies (80). In addition, according to Rosenfeld et al., there is significant difference between BDNF serum and plasma levels, nonetheless as previous reviews, this review also analyzed serum and plasma BDNF levels together (81). Furthermore, in some articles, potential overlap in samples were detected, hence, introducing bias to the analysis. The systematic review also did not account for neither the maturity of BDNF nor BDNF polymorphism, which could play an important role in determining BDNF levels in the periphery. Finally, no studies with exclusively schizoaffective patients were obtained, hence, this patient group is missing from the schizophrenia-bipolar spectrum.
Conclusion
While it has been confirmed that blood BDNF levels, especially during the acute phases are decreased, there are several factors that influence circulating BDNF levels making it unreliable as a biomarker of cognitive dysfunction alone. In contrast, circulating BDNF might be considered as a psychiatric state marker and thus, changes in BDNF levels in the plasma/serum should be evaluated in the context of a wider pattern of risk and protective factors such as inflammatory, immune, and metabolic parameters. Nonetheless, this does not necessarily mean that targeting BDNF would not influence cognition positively. Future research should investigate how different treatments influence circulating BDNF levels and cognitive symptoms, especially executive functioning, and memory, and whether there is a correlation between the changes detected.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
ZD, IS, and PB contributed to conception of the manuscript. ZD wrote the first draft of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of Interest
ZD was an employee of Gedeon Richter Plc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Funding
Recordati S.p.A. provided funds for the open access publication fees.
References
- 1.American Psychiatric Association. American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, TX: American Psychiatric Association; (2013). [Google Scholar]
- 2.WHO. WHO | Schizophrenia. Geneva: WHO; (2018). [Google Scholar]
- 3.Rowland TA, Marwaha S. Epidemiology and risk factors for bipolar disorder. Ther Adv Psychopharmacol. (2018) 8:251–69. 10.1177/2045125318769235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Rheenen TE, Lewandowski KE, Tan EJ, Ospina LH, Ongur D, Neill E, et al. Characterizing cognitive heterogeneity on the schizophrenia-bipolar disorder spectrum. Psychol Med. (2017) 47:1848–64. 10.1017/S0033291717000307 [DOI] [PubMed] [Google Scholar]
- 5.Carruthers SP, Van Rheenen TE, Gurvich C, Sumner PJ, Rossell SL. Characterising the structure of cognitive hetereogenity in schizophrenia spectrum disorder: a systematic review and narrative synthesis. Neurosci Biobehav Rev. (2019) 107:252–78. 10.1016/j.neubiorev.2019.09.006 [DOI] [PubMed] [Google Scholar]
- 6.Torres IJ, Boudreau VG, Yatham LN. Neuropsychological functioning in euthymic bipolar disorder: a meta-analysis. Acta Psychiatr Scand Suppl. (2007) 434:17–26. 10.1111/j.1600-0447.2007.01055.x [DOI] [PubMed] [Google Scholar]
- 7.Vrabie M, Marinescu V, Talaşman A, Tautu O, Drima E, Miclut̨ia I. Cognitive impairment in manic bipolar patients: important, understated, significant aspects. Ann Gen Psychiatry. (2015) 14:41. 10.1186/s12991-015-0080-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldberg JF, Chengappa KNR. Identifying and treating cognitive impairment in bipolar disorder. Bipolar Disord. (2009) 11(Suppl. 2):123–37. 10.1111/j.1399-5618.2009.00716.x [DOI] [PubMed] [Google Scholar]
- 9.Green MF. Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. J Clin Psychiatry. (2006) 67(Suppl. 9):3–8; discussion 36–42. [PubMed] [Google Scholar]
- 10.Trivedi J. Cognitive deficits in psychiatric disorders: current status. Indian J Psychiatry. (2006) 47:10–20. 10.4103/0019-5545.31613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Torrent C, Martínez-Arán A, Amann B, Daban C, Tabarés-Seisdedos R, González-Pinto A, et al. Cognitive impairment in schizoaffective disorder: a comparison with non-psychotic bipolar and healthy subjects. Acta Psychiatr Scand. (2007) 116:453–60. 10.1111/j.1600-0447.2007.01072.x [DOI] [PubMed] [Google Scholar]
- 12.Martinez-Aran A, Vieta E, Torrent C, Sanchez-Moreno J, Goikolea JM, Salamero M, et al. Functional outcome in bipolar disorder: the role of clinical and cognitive factors. Bipolar Disord. (2007) 9:103–13. 10.1111/j.1399-5618.2007.00327.x [DOI] [PubMed] [Google Scholar]
- 13.Bowie CR, Harvey PD. Cognitive deficits and functional outcome in schizophrenia. Neuropsychiatr Dis Treat. (2006) 2:531–6. 10.2147/nedt.2006.2.4.531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaneko K. Negative symptoms and cognitive impairments in schizophrenia: two key symptoms negatively influencing social functioning. Yonago Acta Med. (2018) 61:91–102. 10.33160/yam.2018.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aquila R, Citrome L. Cognitive impairment in schizophrenia: the great unmet need. CNS Spectr. (2015) (Suppl. 1):35–9. 10.1017/S109285291500070X [DOI] [PubMed] [Google Scholar]
- 16.Fernandes BS, Molendijk ML, Köhler CA, Soares JC, Leite CMGS, Machado-Vieira R, et al. Peripheral brain-derived neurotrophic factor (BDNF) as a biomarker in bipolar disorder: a meta-analysis of 52 studies. BMC Med. (2015) 13:289. 10.1186/s12916-015-0529-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peng S, Li W, Lv L, Zhang Z, Zhan X. BDNF as a biomarker in diagnosis and evaluation of treatment for schizophrenia and depression. Discov Med. (2018) 26:127–36. [PubMed] [Google Scholar]
- 18.Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol. (2005) 76:99–125. 10.1016/j.pneurobio.2005.06.003 [DOI] [PubMed] [Google Scholar]
- 19.Gärtner A, Staiger V. Neurotrophin secretion from hippocampal neurons evoked by long-term-potentiation-inducing electrical stimulation patterns. Proc Natl Acad Sci USA. (2002) 99:6386–91. 10.1073/pnas.092129699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindvall O, Kokaia Z, Bengzon J, Elmér E, Kokaia M. Neurotrophins and brain insults. Trends Neurosci. (1994) 17:490–6. 10.1016/0166-2236(94)90139-2 [DOI] [PubMed] [Google Scholar]
- 21.Goff DC. Future perspectives on the treatment of cognitive deficits and negative symptoms in schizophrenia. World Psychiatry. (2013) 12:99–107. 10.1002/wps.20026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Angelucci F, Brenè S, Mathé AA. BDNF in schizophrenia, depression and corresponding animal models. Mol Psychiatry. (2005) 10:345–52. 10.1038/sj.mp.4001637 [DOI] [PubMed] [Google Scholar]
- 23.Erickson KI, Prakash RS, Voss MW, Chaddock L, Heo S, McLaren M, et al. Brain-derived neurotrophic factor is associated with age-related decline in hippocampal volume. J Neurosci. (2010) 30:5368–75. 10.1523/JNEUROSCI.6251-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berry A, Bellisario V, Capoccia S, Tirassa P, Calza A, Alleva E, et al. Social deprivation stress is a triggering factor for the emergence of anxiety- and depression-like behaviours and leads to reduced brain BDNF levels in C57BL/6J mice. Psychoneuroendocrinology. (2012) 37:762–72. 10.1016/j.psyneuen.2011.09.007 [DOI] [PubMed] [Google Scholar]
- 25.Pillai A, Kale A, Joshi S, Naphade N, Raju MSVK, Nasrallah H, et al. Decreased BDNF levels in CSF of drug-naive first-episode psychotic subjects: correlation with plasma BDNF and psychopathology. Int J Neuropsychopharmacol. (2010) 13:535–9. 10.1017/S1461145709991015 [DOI] [PubMed] [Google Scholar]
- 26.Ng TKS, Ho CSH, Tam WWS, Kua EH, Ho RCM. Decreased serum brain-derived neurotrophic factor (BDNF) levels in patients with Alzheimer’s disease (AD): a systematic review and meta-analysis. Int J Mol Sci. (2019) 20:257. 10.3390/ijms20020257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barbosa AG, Pratesi R, Paz GSC, dos Santos MAAL, Uenishi RH, Nakano EY, et al. Assessment of BDNF serum levels as a diagnostic marker in children with autism spectrum disorder. Sci Rep. (2020) 10:1734. 10.1038/s41598-020-74239-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nieto R, Kukuljan M, Silva H. BDNF and schizophrenia: from neurodevelopment to neuronal plasticity, learning, and memory. Front Psychiatry. (2013) 4:45. 10.3389/fpsyt.2013.00045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmed AO, Mantini AM, Fridberg DJ, Buckley PF. Brain-derived neurotrophic factor (BDNF) and neurocognitive deficits in people with schizophrenia: a meta-analysis. Psychiatry Res. (2015) 226:1–13. 10.1016/j.psychres.2014.12.069 [DOI] [PubMed] [Google Scholar]
- 30.Bora E. Peripheral inflammatory and neurotrophic biomarkers of cognitive impairment in schizophrenia: a meta-analysis. Psychol Med. (2019) 49:1971–9. 10.1017/s0033291719001685 [DOI] [PubMed] [Google Scholar]
- 31.Fernandes BS, Gama CS, Maria Ceresér K, Yatham LN, Fries GR, Colpo G, et al. Brain-derived neurotrophic factor as a state-marker of mood episodes in bipolar disorders: a systematic review and meta-regression analysis. J Psychiatr Res. (2011) 25:995–1004. 10.1016/j.jpsychires.2011.03.002 [DOI] [PubMed] [Google Scholar]
- 32.Munkholm K, Vinberg M, Kessing LV. Peripheral blood brain-derived neurotrophic factor in bipolar disorder: a comprehensive systematic review and meta-analysis. Mol Psychiatry. (2016) 21:216–28. 10.1038/mp.2015.54 [DOI] [PubMed] [Google Scholar]
- 33.Moher D, Liberati A, Tetzlaff J, Altman DG, Altman D, Antes G, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Man L, Lv X, Du XD, Yin G, Zhu X, Zhang Y, et al. Cognitive impairments and low BDNF serum levels in first-episode drug-naive patients with schizophrenia. Psychiatry Res. (2018) 263:1–6. 10.1016/j.psychres.2018.02.034 [DOI] [PubMed] [Google Scholar]
- 35.Qu M, Wang J, Chen DC, Chen S, Xiu MH, Zhang XY. Sex-specific association between peripheral superoxide dismutase, BDNF and cognitive impairment in drug-naive first episode patients with schizophrenia. Free Radic Biol Med. (2020) 160:887–93. 10.1016/j.freeradbiomed.2020.09.014 [DOI] [PubMed] [Google Scholar]
- 36.de Azua SR, Matute C, Stertz L, Mosquera F, Palomino A, de la Rosa I, et al. Plasma brain-derived neurotrophic factor levels, learning capacity and cognition in patients with first episode psychosis. BMC Psychiatry. (2013) 13:27. 10.1186/1471-244X-13-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Theleritis C, Fisher HL, Shäfer I, Winters L, Stahl D, Morgan C, et al. Brain derived neurotropic factor (BDNF) is associated with childhood abuse but not cognitive domains in first episode psychosis. Schizophr Res. (2014) 159:56–61. 10.1016/j.schres.2014.07.013 [DOI] [PubMed] [Google Scholar]
- 38.Wu ZW, Shi H, Chen DC, Chen S, Xiu MH, Zhang XY. BDNF serum levels and cognitive improvement in drug-naive first episode patients with schizophrenia: a prospective 12-week longitudinal study. Psychoneuroendocrinology. (2020) 122:104879. 10.1016/j.psyneuen.2020.104879 [DOI] [PubMed] [Google Scholar]
- 39.Xiu MH, Wang DM, Du XD, Chen N, Tan SP, Tan YL, et al. Interaction of BDNF and cytokines in executive dysfunction in patients with chronic schizophrenia. Psychoneuroendocrinology. (2019) 108:110–7. 10.1016/j.psyneuen.2019.06.006 [DOI] [PubMed] [Google Scholar]
- 40.Yang Y, Liu Y, Wang G, Hei G, Wang X, Li R, et al. Brain-derived neurotrophic factor is associated with cognitive impairments in first-episode and chronic schizophrenia. Psychiatry Res. (2019) 273:528–36. 10.1016/j.psychres.2019.01.051 [DOI] [PubMed] [Google Scholar]
- 41.Xiao W, Ye F, Liu C, Tang X, Li J, Dong H, et al. Cognitive impairment in first-episode drug-naïve patients with schizophrenia: relationships with serum concentrations of brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor. Prog Neuro Psychopharmacology Biol Psychiatry. (2017) 76:163–8. 10.1016/j.pnpbp.2017.03.013 [DOI] [PubMed] [Google Scholar]
- 42.Carlino D, Leone E, Di Cola F, Baj G, Marin R, Dinelli G, et al. Low serum truncated-BDNF isoform correlates with higher cognitive impairment in schizophrenia. J Psychiatr Res. (2011) 45:273–9. 10.1016/j.jpsychires.2010.06.012 [DOI] [PubMed] [Google Scholar]
- 43.Vinogradov S, Fisher M, Holland C, Shelly W, Wolkowitz O, Mellon SH. Is serum brain-derived neurotrophic factor a biomarker for cognitive enhancement in schizophrenia? Biol Psychiatry. (2009) 66:549–53. 10.1016/j.biopsych.2009.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wei CW, Sun Y, Chen N, Chen S, Xiu MH, Zhang XY. Interaction of oxidative stress and BDNF on executive dysfunction in patients with chronic schizophrenia. Psychoneuroendocrinology. (2020) 111:104473. 10.1016/j.psyneuen.2019.104473 [DOI] [PubMed] [Google Scholar]
- 45.Wu JQ, Chen DC, Tan YL, Tan SP, Hui L, Lv MH, et al. Altered BDNF is correlated to cognition impairment in schizophrenia patients with tardive dyskinesia. Psychopharmacology (Berl). (2015) 232:223–32. 10.1007/s00213-014-3660-9 [DOI] [PubMed] [Google Scholar]
- 46.Hori H, Yoshimura R, Katsuki A, Atake K, Nakamura J. Relationships between brain-derived neurotrophic factor, clinical symptoms, and decision-making in chronic schizophrenia: data from the Iowa gambling task. Front Behav Neurosci. (2014) 8:417. 10.3389/fnbeh.2014.00417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang XY, Chen DC, Tan YL, Tan SP, Wang ZR, De Yang F, et al. Gender difference in association of cognition with BDNF in chronic schizophrenia. Psychoneuroendocrinology. (2014) 48:136–46. 10.1016/j.psyneuen.2014.06.004 [DOI] [PubMed] [Google Scholar]
- 48.Zhang XY, Liang J, Chen DC, Xiu MH, De Yang F, Kosten TA, et al. Low BDNF is associated with cognitive impairment in chronic patients with schizophrenia. Psychopharmacology (Berl). (2012) 222:277–84. 10.1007/s00213-012-2643-y [DOI] [PubMed] [Google Scholar]
- 49.Chou YH, Wang SJ, Lirng JF, Lin CL, Yang KC, Chen CK, et al. Impaired cognition in bipolar I disorder: the roles of the serotonin transporter and brain-derived neurotrophic factor. J Affect Disord. (2012) 143:131–7. 10.1016/j.jad.2012.05.043 [DOI] [PubMed] [Google Scholar]
- 50.Mora E, Portella MJ, Piñol-Ripoll G, López R, Cuadras D, Forcada I, et al. High BDNF serum levels are associated to good cognitive functioning in bipolar disorder. Eur Psychiatry. (2019) 60:97–107. 10.1016/j.eurpsy.2019.02.006 [DOI] [PubMed] [Google Scholar]
- 51.Rybakowski JK, Suwalska A. Excellent lithium responders have normal cognitive functions and plasma BDNF levels. Int J Neuropsychopharmacol. (2010) 13:617–22. 10.1017/S1461145710000404 [DOI] [PubMed] [Google Scholar]
- 52.Dias VV, Brissos S, Frey BN, Andreazza AC, Cardoso C, Kapczinski F. Cognitive function and serum levels of brain-derived neurotrophic factor in patients with bipolar disorder. Bipolar Disord. (2009) 11:663–71. 10.1111/j.1399-5618.2009.00733.x [DOI] [PubMed] [Google Scholar]
- 53.Dell’Osso L, Bianchi C, Del Debbio A, Roncaglia I, Veltri A, Carlini M, et al. Plasma brain-derived neurotrophic factor in bipolar and unipolar depression. Ital J Psychopathol. (2010) 16:138–43. [Google Scholar]
- 54.Siwek J, Gourlay ML, Slawson DC, Shaughnessy AF. How to write an evidence-based clinical review article. Am Fam Physician. (2002) 65:251–8. [PubMed] [Google Scholar]
- 55.Chang YH, Wang TY, Lee SY, Chen SL, Huang CC, Chen PS, et al. Memory impairment and plasma BDNF correlates of the BDNF Val66Met polymorphism in patients with bipolar II disorder. Front Genet. (2018) 9:583. 10.3389/fgene.2018.00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xiu MH, Li Z, Chen DC, Chen S, Curbo ME, Wu HE, et al. Interrelationships between BDNF, superoxide dismutase, and cognitive impairment in drug-naive first-episode patients with schizophrenia. Schizophr Bull. (2020) 46:1498–510. 10.1093/schbul/sbaa062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang C, Fang X, Yao P, Mao Y, Cai J, Zhang Y, et al. Metabolic adverse effects of olanzapine on cognitive dysfunction: a possible relationship between BDNF and TNF-alpha. Psychoneuroendocrinology. (2017) 81:138–43. 10.1016/j.psyneuen.2017.04.014 [DOI] [PubMed] [Google Scholar]
- 58.Zhang Y, Fang X, Fan W, Tang W, Cai J, Song L, et al. Brain-derived neurotrophic factor as a biomarker for cognitive recovery in acute schizophrenia: 12-week results from a prospective longitudinal study. Psychopharmacology (Berl). (2018) 235:1191–8. 10.1007/s00213-018-4835-6 [DOI] [PubMed] [Google Scholar]
- 59.Asevedo E, Gadelha A, Noto C, Mansur RB, Zugman A, Belangero SIN, et al. Impact of peripheral levels of chemokines, BDNF and oxidative markers on cognition in individuals with schizophrenia. J Psychiatr Res. (2013) 47:1376–82. 10.1016/j.jpsychires.2013.05.032 [DOI] [PubMed] [Google Scholar]
- 60.Hori H, Yoshimura R, Katsuki A, Atake K, Igata R, Konishi Y, et al. Relationships between serum brain-derived neurotrophic factor, plasma catecholamine metabolites, cytokines, cognitive function and clinical symptoms in Japanese patients with chronic schizophrenia treated with atypical antipsychotic monotherapy. World J Biol Psychiatry. (2017) 18:401–8. 10.1080/15622975.2016.1212172 [DOI] [PubMed] [Google Scholar]
- 61.Niitsu T, Shirayama Y, Matsuzawa D, Hasegawa T, Kanahara N, Hashimoto T, et al. Associations of serum brain-derived neurotrophic factor with cognitive impairments and negative symptoms in schizophrenia. Prog Neuro Psychopharmacology Biol Psychiatry. (2011) 35:1836–40. 10.1016/j.pnpbp.2011.09.004 [DOI] [PubMed] [Google Scholar]
- 62.Penadés R, López-Vílchez I, Catalán R, Arias B, González-Rodríguez A, García-Rizo C, et al. BDNF as a marker of response to cognitive remediation in patients with schizophrenia: a randomized and controlled trial. Schizophr Res. (2018) 197:458–64. 10.1016/j.schres.2017.12.002 [DOI] [PubMed] [Google Scholar]
- 63.Xiao J, Mi W, Li L, Shi Y, Zhang H. High relapse rate and poor medication adherence in the chinese population with schizophrenia: results from an observational survey in the people’s Republic of China. Neuropsychiatr Dis Treat. (2015) 11:1161–7. 10.2147/NDT.S72367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dong R, Zhao NO, Wu HE, Yu L, Zhang XY. Sex differences in the association between serum BDNF and cognitive impairment in schizophrenia patients using various antipsychotics. J Psychiatr Res. (2021) 138:429–99. 10.1016/j.jpsychires.2021.04.026 [DOI] [PubMed] [Google Scholar]
- 65.Gold JM, Queern C, Iannone VN, Buchanan RW. Repeatable battery for the assessment of neuropsychological status as a screening test in schizophrenia, I: sensitivity, reliability, and validity. Am J Psychiatry. (1999) 156:1944–50. 10.1176/ajp.156.12.1944 [DOI] [PubMed] [Google Scholar]
- 66.Green MF, Nuechterlein KH, Gold JM, Barch DM, Cohen J, Essock S, et al. Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biol Psychiatry. (2004) 56:301–7. 10.1016/j.biopsych.2004.06.023 [DOI] [PubMed] [Google Scholar]
- 67.Lowe C, Rabbitt P. Test/re-test reliability of the CANTAB and ISPOCD neuropsychological batteries: theoretical and practical issues. Cambridge neuropsychological test automated battery. International study of post-operative cognitive dysfunction. Neuropsychologia. (1998) 36:915–23. 10.1016/s0028-3932(98)00036-0 [DOI] [PubMed] [Google Scholar]
- 68.Keefe RSE, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L. The brief assessment of cognition in schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res. (2004) 1:283–97. 10.1016/j.schres.2003.09.011 [DOI] [PubMed] [Google Scholar]
- 69.Zhang XY, Chen DC, Xiu MH, Haile CN, Luo X, Xu K, et al. Cognitive and serum BDNF correlates of BDNF Val66Met gene polymorphism in patients with schizophrenia and normal controls. Hum Genet. (2012) 131:1187–95. 10.1007/s00439-012-1150-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li S, Chen D, Xiu M, Li J, Zhang XY. Diabetes mellitus, cognitive deficits and serum BDNF levels in chronic patients with schizophrenia: a case-control study. J Psychiatr Res. (2021) 134:39–47. 10.1016/j.jpsychires.2020.12.035 [DOI] [PubMed] [Google Scholar]
- 71.Green MJ, Matheson SL, Shepherd A, Weickert CS, Carr VJ. Brain-derived neurotrophic factor levels in schizophrenia: a systematic review with meta-analysis. Mol Psychiatry. (2011) 16:960–72. 10.1038/mp.2010.88 [DOI] [PubMed] [Google Scholar]
- 72.Fernandes BS, Steiner J, Berk M, Molendijk ML, Gonzalez-Pinto A, Turck CW, et al. Peripheral brain-derived neurotrophic factor in schizophrenia and the role of antipsychotics: meta-analysis and implications. Mol Psychiatry. (2015) 20:1108–19. 10.1038/mp.2014.117 [DOI] [PubMed] [Google Scholar]
- 73.Fernandes BS, Berk M, Turck CW, Steiner J, Gonçalves CA. Decreased peripheral brain-derived neurotrophic factor levels are a biomarker of disease activity in major psychiatric disorders: a comparative meta-analysis. Mol Psychiatry. (2014) 19:749–51. 10.1038/mp.2013.172 [DOI] [PubMed] [Google Scholar]
- 74.Bath KG, Schilit A, Lee FS. Stress effects on BDNF expression: effects of age, sex, and form of stress. Neuroscience. (2013) 239:149–56. 10.1016/j.neuroscience.2013.01.074 [DOI] [PubMed] [Google Scholar]
- 75.Miranda M, Morici JF, Zanoni MB, Bekinschtein P. Brain-derived neurotrophic factor: a key molecule for memory in the healthy and the pathological brain. Front Cell Neurosci. (2019) 13:363. 10.3389/fncel.2019.00363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Razgado-Hernandez LF, Espadas-Alvarez AJ, Reyna-Velazquez P, Sierra-Sanchez A, Anaya-Martinez V, Jimenez-Estrada I, et al. The transfection of BDNF to dopamine neurons potentiates the effect of dopamine D3 receptor agonist recovering the striatal innervation, dendritic spines and motor behavior in an aged rat model of Parkinson’s disease. PLoS One. (2015) 10:e0117391. 10.1371/journal.pone.0117391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guillin O, Diaz J, Carroll P, Griffon N, Schwartz JC, Sokoloff P. BDNF controls dopamine D3 receptor expression and triggers behavioural sensitization. Nature. (2001) 411:86–9. 10.1038/35075076 [DOI] [PubMed] [Google Scholar]
- 78.Griffon N, Sokoloff P, Diaz J, Lévesque D, Sautel F, Schwartz JC, et al. The dopamine D3 receptor and schizophrenia: pharmacological, anatomical and genetic approaches. Eur Neuropsychopharmacol. (1995) 5(Suppl.):3–9. 10.1016/0924-977x(95)00030-s [DOI] [PubMed] [Google Scholar]
- 79.Mitchell P, Waters B, Vivero C, Le F, Donald J, Tully M, et al. Exclusion of close linkage of bipolar disorder to the dopamine D3 receptor gene in nine Australian pedigrees. J Affect Disord. (1993) 27:213–24. 10.1016/0165-0327(93)90045-l [DOI] [PubMed] [Google Scholar]
- 80.Field AP. Meta-analysis of correlation coefficients: a Monte Carlo comparison of fixed- and random-effects methods. Psychol Methods. (2001) 6:161–80. 10.1037/1082-989x.6.2.161 [DOI] [PubMed] [Google Scholar]
- 81.Rosenfeld RD, Zeni L, Haniu M, Talvenheimo J, Radka SF, Bennett L, et al. Purification and identification of brain-derived neurotrophic factor from human serum. Protein Expr Purif. (1995) 6:465–71. 10.1006/prep.1995.1062 [DOI] [PubMed] [Google Scholar]
- 82.Tang X, Zhou C, Gao J, Duan W, Yu M, Xiao W, et al. Serum BDNF and GDNF in Chinese male patients with deficit schizophrenia and their relationships with neurocognitive dysfunction. BMC Psychiatry. (2019) 19:254. 10.1186/s12888-019-2231-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.