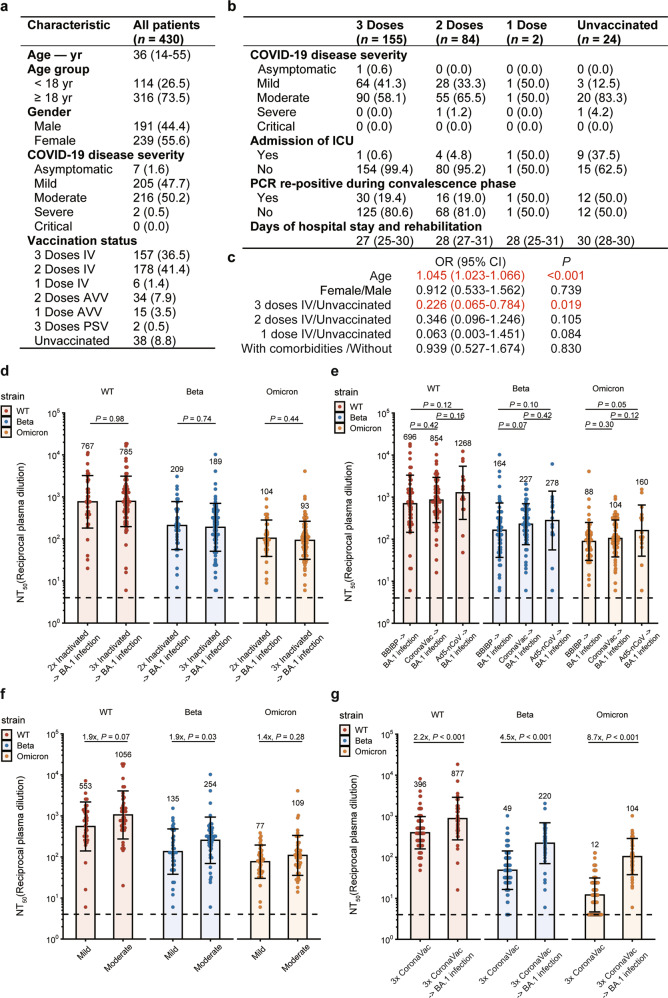
Fig. 1. Protection of inactivated vaccine against Omicron BA.1.
a Characteristics of Omicron-infected patients. Continuous variables were shown in median (IQR) and categorical variables were summarized as counts (percentages). COVID-19 disease severity was defined according to WHO living guidance for clinical management of COVID-19.10 IV, inactivated vaccine; AVV, adenovirus-vectored vaccine; PSV, recombinant protein subunit vaccine. b Correlation between inactivated vaccine doses and COVID-19 disease severity and progression in adult patients. PCR re-positive during convalescence phase was defined as PCR Ct value less than 40 after two independent PCR-negative results with an interval of more than 24 h. c Multivariate analysis of risk and protective factors for COVID-19 disease severity in Omicron-infected adult individuals. Statistical significance was determined by ordered multi-class logistic regression. d Humoral immune responses against WT and variants of SARS-CoV-2 among breakthrough Omicron convalescents. The geometric mean titer (GMT), geometric standard deviation, and fold-changes of 50% neutralization titers (NT50) are labeled. Dashed lines show the limit of detection. Statistical significance was determined by a two-tailed Wilcoxon test. e NT50 from the breakthrough BA.1 convalescents who were vaccinated with BBIBP-CorV (n = 54), CoronaVac (n = 65), or Ad5-nCoV (n = 16) vaccine. The plasma titers refer to all the recipients of the same type of vaccine, including 1-dose, 2-dose, and 3-dose vaccines. f NT50 from the breakthrough BA.1 convalescents with mild (n = 36) or moderate (n = 43) symptoms after 3 doses of inactive vaccine immunization. g NT50 from the breakthrough BA.1 convalescents who were vaccinated with 3 doses of CoronaVac vaccine (n = 42) or from the uninfected vaccinees who had matched vaccination profiles (n = 114).