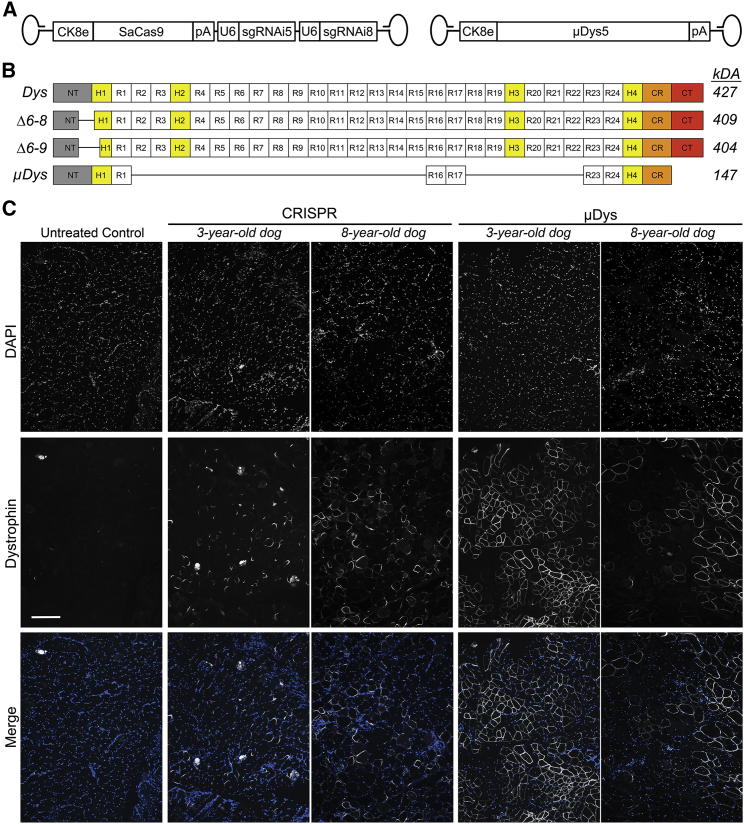
Figure 2.
Induction of dystrophin expression following AAV-mediated CRISPR/Cas9 gene editing and micro-dystrophin gene replacement
(A) Overview of AAV6 vectors expressing SaCas9 under control of the CK8e regulatory cassette and two single gRNA cassettes targeting introns 5 and 8 of the canine dystrophin gene as well as micro-dystrophin-5 (μDys5) under control of CK8e. (B) Schematics depicting structural domains present in normal full-length dystrophin (427 kDa), Δ6–8 dystrophin (∼409 kDa), Δ6–9 dystrophin (∼404 kDa), and μDys5 (147 kDa). (C) Immunofluorescence analysis of well-transduced muscle sample regions from the 3- and 8-year-old dogs injected with AAV6:CK8e-CRISPR/Cas9 (CRISPR) and AAV6:CK8e-μDys5 (μDys). Untreated control samples were derived from non-treated vastus lateralis muscles. Sections were stained with DAPI (top row) and for dystrophin (middle row). Merged images of DAPI and dystrophin are shown in the bottom row. CRISPR-treated sections were stained with antibodies raised against the C-terminus of dystrophin, while untreated control- and μDys-treated samples were stained with antibodies raised against the hinge 1/spectrin-like repeat 1 (H1/R1) region of dystrophin, as μDys5 contains this region but lacks the majority of the C-terminus. Scale bar, 100 μm. Images of control wild-type cranial tibialis muscle sections stained with both C-terminal and H1/R1 dystrophin antibodies are available in Figure S1.