Abstract
Vietnam has been identified as a country at high-risk for emergence and re-emergence of zoonotic diseases. The government of Vietnam recognized five priority zoonoses, including highly pathogenic avian influenza, rabies, leptospirosis, anthrax, and Streptococcus suis, and established a framework for One Health investigation and response to these diseases. From July 2020 to February 2021, quantitative data of zoonoses were collected from an online survey in 61 of 63 provinces based on either clinical diagnosis or laboratory confirmation. The responses were followed up by using in-depth interviews, and scientific literatures on zoonoses in Vietnam during 2010 to 2020 were reviewed. A total of 234 human health professionals and 95 animal health professionals responded to the survey. The proportion of clinical-based respondents was higher than laboratory-based respondents in both human health (130/234, 55.6%) and animal health (65/95, 68.4%) sectors. There were differences in the reported frequency of zoonoses between human and animal health professionals, and between clinical-based and laboratory-based respondents. Rabies was the most serious zoonotic disease based on the number of human cases and the geographic distribution. No human cases of avian influenza infection have been reported since 2015, although the H5 subtype viruses have been found in poultry. Besides, some bacterial, fungal, and parasitic zoonoses were detected in both humans and animals. Out of the 75 zoonoses identified, we recommend that the original five prioritized zoonoses, plus 24 additional zoonoses, should be targeted for future prevention, detection, and control under One Health approach in Vietnam.
Keywords: Zoonoses, Survey, Prioritization, One health, Vietnam
1. Introduction
In recent years, global public health security has been threatened by the emergence and re-emergence of zoonotic diseases, as exemplified by outbreaks of Ebola virus, avian influenza viruses, severe acute respiratory syndrome (SARS) coronavirus, and the Middle East respiratory syndrome (MERS) coronavirus [1]. The 2019 outbreaks of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), likely caused by animal species crossing to infect humans, have become a serious public and global health threat [1].
The increase of emergence and re-emergence of zoonotic diseases has been driven by increasing human and animal populations, infringement of wildlife habitats, the growing demand for wildlife and wildlife products, changing farming practices, climate change, and ease and speed of global travel [2]. Vietnam is a country at risk for emergence and re-emergence of zoonotic diseases due to high human and livestock densities, increasing urbanization, large volume of domestic as well as cross-border trades for animals and animal products, prevailing small-scale livestock production, poor biosecurity practices, and mixed farming systems [3].
Highly pathogenic avian influenza, rabies, anthrax, leptospirosis and Streptococcus suis (S. suis) have been commonly reported in Vietnam [4]. In 2015, a study was conducted to establish strategic priorities for zoonotic disease control in Vietnam, and 5 out of 12 diseases were selected for prioritization including avian influenza, rabies, S. suis, pandemic influenza and foodborne bacterial diseases [5].
To mitigate the risk of emergence and re-emergence of zoonotic diseases, the government of Vietnam issued a joint circular number 16 in 2013 [6] providing the guidelines for a multisectoral coordinated investigation and response to zoonotic diseases and specified Vietnam priority zoonoses using the One Health approach [7]. In subsequent years, both the Ministry of Health [8] and Ministry of Agriculture and Rural Development [9] issued regulations to support surveillance and reporting of these prioritized zoonoses and to strengthen coordination, information sharing, and collaboration between human health and animal health sectors.
Identifying zoonotic pathogens/diseases commonly detected in Vietnam can provide critical evidence to support further investment in the One Health coordinated zoonotic diseases surveillance and effective prevention and control measures in both humans and animals. Specifically, we identified what zoonoses have been detected, and estimated their frequency of detection in Vietnam.
2. Methodology
2.1. Study design
A retrospective mixed method study was conducted from July 2020 to February 2021, in which quantitative data were gathered from an online survey, the responses were followed up using in-depth interviews, and a literature review of zoonotic pathogens/diseases in Vietnam was analyzed.
The online survey was implemented from July to September 2020. For human health sector, at the central level, all general hospitals, specialized hospitals, medical research institutes, and laboratories related to zoonotic diseases were invited to respond to our questionnaires. At the regional level, we included the National Institute of Hygiene and Epidemiology (NIHE), the Pasteur Institute of Nha Trang, the Pasteur Institute of Ho Chi Minh city (PI– HCMC), and Tay Nguyen Institute of Hygiene and Epidemiology. At the local level, we surveyed provincial general hospitals and all provincial Centres for Disease Control.
For animal health, at the central level, all veterinary research institutes, universities, companies related to animal health and national veterinary laboratories were selected. Seven Regional Animal Health Offices (RAHOs) were included as the regional level. At the local level, the provincial Sub-Departments of Animal Health, local veterinary laboratories, zoos and safari, wildlife rescue centres, and livestock production companies having laboratory diagnostic capacity were also recruited in the survey.
2.2. Selection of respondents
Individuals working for the human health or animal health sectors, either as physicians or medical, laboratory or technical staff in the selected entities were eligible for their participation in the survey. We assumed receiving at least two respondents from all the shortlisted entities either clinical-based or laboratory-based staff to fill in the questionnaires, plus departments involved in research on parasitic pathogens/diseases in animals or humans.
2.3. Questionnaire design
To select the survey items, we initially reviewed a list of potential global zoonotic pathogens/diseases from the MSD manual [10], the book ‘Diseases That Can Spread Between Animals and People’ [11], and information from Public Health England [12]. The inclusion criteria were zoonotic pathogens/diseases and their potential hosts and reservoirs previously identified either globally, in Asia, in Southeast Asia region or in Vietnam, and neglected zoonoses transmitted between humans and vertebrate animals.
Three types of questionnaires were developed for three different groups of respondents. Clinical-based respondents in animal health and human health (Survey BM01) were asked for: (1) any suspected or treated case(s) of zoonotic diseases during last five years; (2) an estimation of the average number of cases for one year; (3) indication of laboratory confirmation or suspected cases. If a laboratory confirmation was verified, the number of cases of each specific zoonotic disease in the last five years was investigated. Laboratory-based respondents in animal health and human health (Survey BM02) were asked about the number of cases/samples for zoonosis testing, the number of positive cases in every year during the last five years. Respondents who conducted research on parasitic pathogens/diseases in animal health and human health (Survey BM03) were interviewed on the number of cases/samples and the number of positives recorded in the last five-year period. The pre-tested questionnaires were then designed as a web-based online survey, using Kobo-toolbox [13].
2.4. Survey follow-up activities
The initial data analysis was undertaken between October and November 2020. A team consisting of a specialist in qualitative research from Oxford University Clinical Research Unit (OUCRU) in Ho Chi Minh city and other members from the Food and Agriculture Organization (FAO), Department of Animal Health (DAH), NIHE, PI–HCMC, and USAID followed up in-depth interviews to the participants who provided information on the frequency of detection and identification of zoonotic pathogens/diseases in the questionnaires (BM01, BM02, or BM03). Other respondents not participating in the online survey received a shorter version of the survey asking for the number of times of detection of any zoonosis.
2.5. Literature review
A literature review was implemented from December 2020 to February 2021 by searching for publications related to zoonotic diseases in Vietnam from 2010 to 2020 through PubMed.
2.6. Data management and analysis
The Kobo-toolbox application automatically recorded the online response data. Besides, the answers of the respondents from the follow-up activities and those who completed the short form of the survey were also entered in the application. The data were downloaded into MS-Excel, then were transferred to MS-Access. The frequency of pathogens/diseases was estimated from the total number of responses. The literature review information was summarized in MS-Excel tables. We assigned a score for each pathogen/disease according to the number of cases reported in the literatures as: 0 = no report; + = 1–3 cases; ++ = 4–10 cases; and +++≥10 cases. To select pathogens/diseases for prioritization, we determined the relative significance of those identified by combining information from the online survey and the literature review into an index based on history of detection (frequency, host, identification methods) weighted by the expected public health impact of the outbreaks. The weighted index ranged from 0, +, ++ and +++. Diseases/pathogens with weighted index ++ and +++ were considered as a significance for future detection, prevention, and control in addition to the current prioritized zoonotic diseases by the government.
2.7. Ethical considerations
To protect the confidentiality of the respondents in the on-line survey, and those who took part in follow-up activities, it was stated that no information linked to respondents would be publicized without their consent and that participation in the survey was voluntary.
3. Results
We shortlisted 397 entities to participate in the online survey. Of these, 36 were unable to connect to internet, or did not receive a letter of invitation. An additional 153 did not participate, citing their busy schedules or no information to share.
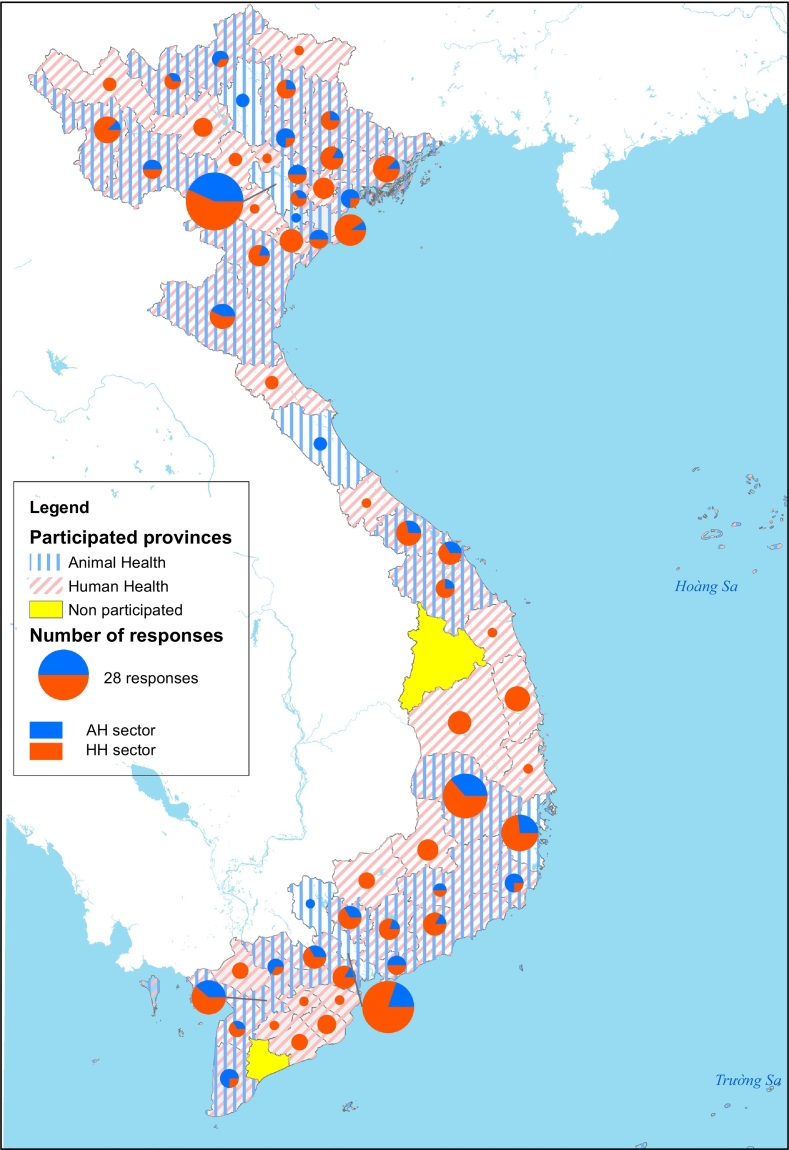
A total of 209 entities responded to our questionnaires, of which 61 were from animal health sector and 148 were from human health sector (Fig. 1). The response rate by organisations was 57.9% (209/361). The total respondents in this survey were 332. The proportion of clinical-based respondents was higher than laboratory-based respondents in both human health (138/237, 58.2%) and animal health (66/95, 69.4%) professionals (Table 1).
Fig. 1.
Number of respondents to the online survey by province, July–November 2020, Vietnam.
Table 1.
Online survey by sector, entity and response, linked to animal or human health and clinical or laboratory/research respondents, July–November 2020, Vietnam.
| Sector |
Entity type |
Entities/Institutions |
Responses |
||||
|---|---|---|---|---|---|---|---|
| Shortlisted | Sent | Returned, checked & verified | Total responses | Clinical (BM01) | Laboratory & Research (BM02/BM03) | ||
| Animal Health (AH) | Company | 11 | 11 | 3 | 3 | 2 | 1 |
| Education/Research | 7 | 7 | 3 | 5 | 3 | 2 | |
| Epi-unit | 2 | 2 | 2 | 6 | 3 | 3 | |
| Clinical Laboratory | 3 | 3 | 2 | 3 | 1 | 2 | |
| RAHOa | 7 | 7 | 7 | 13 | 6 | 7 | |
| SDAHb | 63 | 63 | 37 | 58 | 46 | 12 | |
| Zoo/wildlife | 14 | 14 | 7 | 7 | 5 | 2 | |
| Sum of AH sector | 107 | 107 | 61 | 95 | 66 | 29 | |
| Human Health (HH) | Central General Hospital | 10 | 10 | 5 | 5 | 4 | 1 |
| Education/Research | 2 | 1 | 1 | 3 | 3 | ||
| Epi-Institute | 13 | 12 | 7 | 16 | 6 | 10 | |
| PCDCc | 83 | 65 | 39 | 59 | 33 | 26 | |
| PGHd | 83 | 77 | 23 | 35 | 17 | 18 | |
| Sector's General Hospital | 23 | 22 | 21 | 40 | 21 | 19 | |
| Specialized Hospital | 76 | 67 | 52 | 79 | 57 | 22 | |
| Sum of HH sector | 290 | 254 | 148 | 237 | 138 | 99 | |
| Total | 397 | 361 | 209 | 332 | 204 | 128 | |
RAHO: Regional Animal Health Office.
SDAH: Provincial Sub-Department of Animal Health.
PCDC: Provincial Center for Disease Control.
PGH: Provincial General Hospital.
The location of the 209 entities responded was disaggregated by provinces. Of 63 provinces, 61 were represented, with only Kon Tum and Bac Lieu provinces abstaining. The location of responses was summarized based on the four epidemiological regions of human health sector, linked to the seven regional animal health offices (Table 2).
Table 2.
Responses to the online survey by epidemiological regions, regional animal health offices and provinces, July–November 2020, Vietnam.
| EPI regions | RAHOse | Total provinces | Number of responses |
||||
|---|---|---|---|---|---|---|---|
| By province | By organization/entity | By individual | Animal health | Human health | |||
| Region 1a (Northern) | RAHO1 | 12 | 12 | 49 | 73 | 23 | 50 |
| RAHO2 | 13 | 13 | 35 | 59 | 18 | 41 | |
| RAHO3 | 3 | 3 | 12 | 14 | 4 | 10 | |
| Region 2b (South Central Coast) | RAHO3 | 3 | 3 | 7 | 10 | 4 | 6 |
| RAHO4 | 6 | 6 | 23 | 34 | 7 | 27 | |
| RAHO6 | 2 | 2 | 5 | 10 | 4 | 6 | |
| Region 3c (Highland) | RAHO5 | 4 | 3 | 16 | 33 | 8 | 25 |
| Region 4d (Southern) | RAHO5 | 1 | 1 | 2 | 2 | 1 | 1 |
| RAHO6 | 9 | 9 | 38 | 62 | 15 | 47 | |
| RAHO7 | 10 | 9 | 22 | 35 | 11 | 24 | |
| Total | 63 | 61 | 209 | 332 | 95 | 237 | |
Region 1: National Institute for Hygiene and Epidemiology (NIHE).
Region 2: Pasteur Institute in Nha Trang (PI–Nha Trang).
Region 3: Tay Nguyen Institute for Hygiene and Epidemiology (TIHE).
Region 4: Pasteur Institute in Ho Chi Minh city (PI–HCMC).
RAHOs: Regional Animal Health Offices.
Regarding the current five prioritized zoonotic diseases, for highly pathogenic avian influenza (HPAI), there were 127 human cases of H5N1 virus infection during 2003–2014, of which 63 were fatal. However, there has been not any case reported since 2015, although the H5 subtype viruses have been frequently detected in domestic bird population. According to a report of DAH, 84 communes in 28 provinces notified outbreaks of HPAI and 255,209 poultry were culled in 2020. Two subtypes of HPAI strains, including H5N1 and H5N6, were confirmed in these outbreaks.
Rabies was the most dangerous disease in Vietnam causing the highest number of deaths compared to other infectious diseases in humans. There was an average of 88 human cases and 398,545 dog-bite victims per year during 2012–2016, and fewer 76 fatal cases but higher 510,913 dog-bite cases per year in the period from 2017 to 2021. More human cases were found in the northern, central, and highland regions. Between 2017 and 2021, 2068 dog head samples taken from 35 provinces were tested, of which 227 (10.98%) provided positive results for rabies.
Annual incidence rate of Leptospira was estimated at 0.05–0.25 per 100,000 during 2002–2011, including 369 laboratory confirmed cases with no deaths. Of the 25 serogroups circulating in Vietnam, serogroups Hebdomadis, Pomona, Saxkoebing, and Panama were the most common. From 2014 to 2016, 5 provinces reported a thousand pigs getting leptospirosis. However, Leptospira has not been detected in humans as well as in animals since 2017.
From 2006 to 2011, there were 413 patients of anthrax including 3 fatal cases. During 2012–2022, a total of 266 human cases were confirmed in six mountainous provinces in the northwest region, with no deaths. Between 2020 and 2021, 7 livestock were infected by anthrax in the northwest region.
About 55 to 173 people were hospitalized per year because of S. suis type 2 during the period from 2011 to 2018. The morbidity rate was highest in 2017 with 0.19 cases per 100,000. In domestic pigs, the infection rate varied from 0% to 85.19% during 2011–2019.
The online survey results indicated that six bacteria were detected in both humans and animals, which are Campylobacter, Clostridium, E. coli, Methicillin-resistant Staphylococcus aureus (MRSA), Salmonella, and Vibrio parahemoliticus. However, cat-scratch disease, erysipelas, and pasteurellosis were found only in animals. In contrast, melioidosis and V. cholera were diagnosed only in humans. There were very few reports of brucellosis, Mycobacterium avium, listeriosis, and rat-bite fever. In addition, reports of cases of Acrobacter, Lyme disease, chlamydiosis, glanders, M. bovis and plague were not confirmed by the laboratory testing. Furthermore, Q-fever was reported by 8 laboratory-based respondents, but only three cases had laboratory confirmation.
Four fungi and rickettsia diseases were recorded in both humans and animals, which are aspergillosis, ringworm, typhus, and ehrlichiosis. Five fungal diseases were detected only in humans including cryptococcosis, histoplasmosis, Malassezia infection, penicilliosis, and sporotrichosis.
Twelve zoonoses caused by protozoa and helminths were found in both humans (as larva migrans) and in animals (as adult worms), which are balantidiasis, cryptosporidiosis, giardiasis, toxoplasmosis, fascioliasis, clonorchiasis, dicrocoeliasis, paragonimiasis, cysticercosis, Asian taeniasis, trichostrongyliasis, and trichuriasis. Whereas six diseases were detected only in animals including diphyllobothriasis, raillietiniasis, beef tapeworm disease, ascariasis, ancylostomiasis, and oesophagostomiasis. Both dracunculiasis and dirofilariasis were detected only in humans.
Four viruses were reported in both humans and animals including HPAI, rabies, Japanese encephalitis B, and hepatitis E. However, diseases caused by the H1N1 subtype virus and zika virus were reported in humans only. Additionally, the online survey respondents reported human cases of herpes B and SARS. In contrast, there were no reports of Newcastle disease or foot and mouth disease in humans, even they are the diseases transmissible to humans. Some reports of chikungunya, Nipah virus in bats, hantavirus, and coronaviruses were also recorded in this online survey.
In total, we reviewed 107 publications for zoonoses in Vietnam during 2010–2020 (Table 3). Of the original 112 zoonotic pathogens/diseases from the literature review, we identified 75 to be recorded from the online survey, including 22 bacterial, 2 rickettsia, 4 fungal, 38 parasitic, and 9 viral zoonoses. Furthermore, our study illustrated that 26 zoonoses achieved 1 to 3 publications, 19 had 4 to 10 publications, and 11 were found with more than 10 publications.
Table 3.
Research published between 2010 and February 2020 linked to zoonoses reported in the online survey, December 2020–February 2021, Vietnam.
| Reports on zoonotic pathogens/diseases |
|||||
|---|---|---|---|---|---|
| Publication | 0a | +b | ++c | +++d | Sum |
| 0a | 35 | 7 | 8 | 2 | |
| +b | 10 | 6 | 6 | 1 | |
| ++c | 5 | 12 | 1 | 5 | |
| +++d | 1 | 1 | 4 | 3 | |
| Total | 51 | 26 | 19 | 11 | 107 |
0: no report available.
1–3 cases.
4–10 cases.
>10 cases.
In addition to the original five priority zoonoses, this study identified 24 additional zoonoses commonly detected in Vietnam during last 10 years (Table 4). These findings can benefit from investigation and response based on their relative significance in terms of One Health application (Table 5).
Table 4.
Frequency of common zoonoses reported by online survey and literature review, Vietnam, 2020–2021.
| Agent | Zoonoses | Number of time reported by laboratories | Number of time reported by clinicians | Level of human cases reported in the literature | Level of animal cases reported in the literature | |
|---|---|---|---|---|---|---|
| 1 | Bacteria | Methicillin-resistant Staphylococcus aureus (MRSA) | 25 | 58 | +++ | ++ |
| 2 | Fungal | Aspergillosis | 15 | 60 | +++ | ++ |
| 3 | Bacteria | Brucellosis | 2 | 17 | + | 0 |
| 4 | Bacteria | Campylobacter enteritis | 4 | 20 | ++ | ++ |
| 5 | Bacteria | Whitmore | 0 | 0 | ++ | + |
| 6 | Bacteria | Clostridial diseases | 11 | 34 | ++ | +++ |
| 7 | Bacteria | Enterohemorrhagic E. coli infection | 12 | 89 | ++ | + |
| 8 | Bacteria | Salmonellosis | 33 | 75 | ++ | +++ |
| 9 | Bacteria | Vibriosis-V. cholera | 0 | 0 | ++ | + |
| 10 | Bacteria | Vibriosis-V. parahaemolyticus | 7 | 30 | ++ | +++ |
| 11 | Rickettsia | Typhus | 0 | 0 | ++ | + |
| 12 | Rickettsia | Spotted fever | 0 | 0 | ++ | + |
| 13 | Rickettsia | Scrub typhus | 0 | 0 | ++ | + |
| 14 | Helminth | Clonorchiasis | 16 | 28 | ++ | +++ |
| 15 | Helminth | Fascioliasis | 8 | 14 | ++ | +++ |
| 16 | Helminth | Cysticercosis | 26 | 49 | +++ | +++ |
| 17 | Helminth | Sparganosis | 0 | 9 | + | + |
| 18 | Helminth | Cutaneous larva migrans (ancylostomiasis) | 18 | 41 | + | +++ |
| 19 | Helminth | Trichostrongyliasis | 24 | 48 | +++ | +++ |
| 20 | Helminth | Trichuriasis | 9 | 22 | +++ | +++ |
| 21 | Virus | Hepatitis E | 7 | 26 | + | ++ |
| 22 | Virus | Japanese encephalitis (B) | 21 | 65 | +++ | + |
| 23 | Virus | Hantavirus hemorrhagic | 1 | 4 | + | ++ |
| 24 | Virus | Zika | 0 | 0 | ++ | 0 |
Table 5.
Proposed targeted 24 zoonoses from online survey and literature review, July 2020–January 2021, Vietnam.
| Agent | Zoonoses | History of circulation (2010−2020) | Cases reported (2015–2020) | Suspected cases (2015–2020) | Confirmed cases (2015–2020) | Human cases (2010–2020) | Impact if detected | Weighted Index | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Bacteria | Methicillin-resistant S. aureus (MRSA) | ++ | ++ | +++ | ++ | +++ | +++ | +++ |
| 2 | Fungal | Aspergillosis | +++ | ++ | ++ | ++ | +++ | ++ | +++ |
| 3 | Bacteria | Brucellosis | + | + | + | ++ | ++ | ++ | ++ |
| 4 | Bacteria | Campylobacter enteritis | ++ | ++ | + | ++ | ++ | + | ++ |
| 5 | Bacteria | Whitmore | ++ | + | ++ | ++ | + | +++ | ++ |
| 6 | Bacteria | Clostridial diseases | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| 7 | Bacteria | Enterohemorrhagic E. coli infection | ++ | ++ | +++ | ++ | ++ | ++ | ++ |
| 8 | Bacteria | Salmonellosis | ++ | ++ | +++ | ++ | ++ | ++ | ++ |
| 9 | Bacteria | Vibriosis-V. parahaemolyticus | +++ | ++ | ++ | ++ | ++ | ++ | ++ |
| 10 | Ricketsia | Typhus | + | ++ | ++ | ++ | ++ | ++ | ++ |
| 11 | Ricketsia | Spotted fever | + | ++ | ++ | ++ | ++ | ++ | ++ |
| 12 | Ricketsia | Scrub typhus | + | ++ | ++ | ++ | ++ | ++ | ++ |
| 13 | Protozoa | Toxoplasmosis | ++ | ++ | ++ | + | + | + | ++ |
| 14 | Helminth | Clonorchiasis | ++ | ++ | + | ++ | ++ | ++ | ++ |
| 15 | Helminth | Fasciolopsiasis | ++ | ++ | + | ++ | + | + | ++ |
| 16 | Helminth | Fascioliasis | ++ | ++ | ++ | + | ++ | + | ++ |
| 17 | Helminth | Cysticercosis | +++ | ++ | ++ | + | ++ | ++ | ++ |
| 18 | Helminth | Sparganosis | ++ | ++ | + | + | + | ++ | ++ |
| 19 | Helminth | Cutaneous larva migrans (ancylostomiasis) | + | ++ | ++ | + | ++ | + | ++ |
| 20 | Helminth | Trichostrongyliasis | ++ | + | ++ | ++ | ++ | + | ++ |
| 21 | Helminth | Trichuriasis | ++ | + | ++ | ++ | ++ | +++ | ++ |
| 22 | Virus | Hepatitis E | ++ | + | + | ++ | + | ++ | ++ |
| 23 | Virus | Japanese encephalitis (B) | ++ | + | + | ++ | +++ | ++ | ++ |
| 24 | Virus | Zika | + | + | +++ | + | ++ | +++ | ++ |
4. Discussion and conclusions
The objective of this online survey was to assess the frequency of circulating zoonotic pathogens/diseases in Vietnam that could be targeted for multisectoral collaboration and cooperation following One Health approach. A previous attempt to prioritize zoonoses in Vietnam conducted in 2015 provided a list of 12 zoonotic diseases (5). In this study, of the 75 reported zoonotic diseases, we identified 24 additional zoonoses commonly detected in Vietnam during last decade in addition to the five current prioritized zoonoses.
Several other countries have conducted similar studies. We used a common methodology of generating lists of pathogens/diseases to be prioritized based on expert consultation and review of literature and then determining their significance based on disease frequencies in the population [[14], [15], [16], [17], [18], [19], [20]]. Prioritization criteria commonly include measures of disease burden or frequency such as prevalence or incidence [19,20]. However, if incidence or prevalence data are not readily available, especially in low-income countries, other proxy measures have been used such as epidemic scales [14]. Our study generated a measure of frequency that was used as one criterion for prioritization. The list of common zoonotic diseases in Vietnam was similar to other countries. For instance, rabies, influenza, and brucellosis were commonly included in the list of priority zoonoses in Asia [14,17], and Africa [16,18,20].
Our analysis of the online survey identified a difference between the responses of the clinical and the laboratory groups. While the laboratory analysis confirmed the prevalence of a pathogen/disease with fewer positive cases, the clinical diagnosis provided broader data on suspected cases that can support a basis for laboratory diagnosis. To maximize the probability of identifying the presence of zoonotic pathogens/diseases in this study, we combined both laboratory and clinical examination results into the level of cases observed.
The survey was conducted during COVID-19 pandemic in Vietnam which may resulted in limited number of responses. However, the online survey data received responses from provincial agencies in every region. We could estimate frequency of zoonoses based on the number of responses, but we were unable to estimate the total number of populations to calculate the incidence. We circulated the invitation and the surveys to the entities with no control over the responders who may not be aware of the disease situation or only aware of some pathogens. A review of hospital and laboratory records for zoonoses can help validate our findings.
Additional surveillance efforts on the 24 identified zoonotic diseases in this study can shed lights on the roles of animal reservoir and their public health impact. A One Health multisectoral response to the detection of these zoonoses can also reduce the burden of the zoonotic diseases in Vietnam.
Funding
This work was conducted with financial support from the United States Agency for International Development (USAID) grant number GHA-G-00-06-00001 through the Emergency Centre for Transboundary Animal Diseases (ECTAD), Food and Agriculture Organization of the United Nations (FAO), Country Office for Vietnam.
CRediT authorship contribution statement
Long Pham-Thanh: Conceptualization, Methodology, Data curation, Validation, Writing – original draft. Thu Van Nhu: Conceptualization, Methodology, Investigation, Data curation, Validation, Writing – review & editing. Trung Vinh Nguyen: Methodology, Software, Data curation, Visualization. Khang Vuong Tran: Data curation, Investigation, Validation. Khanh Cong Nguyen: Conceptualization, Methodology, Data curation, Investigation. Huong Thi Nguyen: Conceptualization, Methodology. Hoa Thi Ngo: Conceptualization, Methodology, Investigation, Validation. Pawin Padungtod: Conceptualization, Methodology, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare no Conflicts of Interests.
Acknowledgements
We appreciate the strong commitment and support from DAH, GDPM, NIHE and OUCRU staff members for supporting the development of the questionnaire and collecting and analysing data. We thank Dorothy L Southern, the Manuscript and Publication Editor of FAO, for his critical review of this manuscript.
Contributor Information
Long Pham-Thanh, Email: longpt.cty@mard.gov.vn.
Thu Van Nhu, Email: VanThu.Nhu@fao.org.
Trung Vinh Nguyen, Email: trungnv@oucru.org.
Khang Vuong Tran, Email: khangtv@oucru.org.
Khanh Cong Nguyen, Email: nck@nihe.org.vn.
Huong Thi Nguyen, Email: huong.fetp@gmail.com.
Ngo Thi Hoa, Email: hoant@oucru.org.
Pawin Padungtod, Email: Pawin.Padungtod@fao.org.
References
- 1.Editorial Emerging zoonoses: A one health challenge. EClinical Medicine. Lancet. 2020 doi: 10.1016/j.eclinm.2020.100300. Editorial| Volume 19, 100300, February 01. [DOI] [Google Scholar]
- 2.IPBES, Daszak P., Amuasi J., das Neves C.G., Hayman D., Kuiken T., Roche B., Zambrana-Torrelio C., Buss P., Dundarova H., Feferholtz Y., Földvári G., Igbinosa E., Junglen S., Liu Q., Suzan G., Uhart M., Wannous C., Woolaston K., Mosig Reidl P., O’Brien K., Pascual U., Stoett P., Li H., Ngo H.T. IPBES secretariat; Bonn, Germany: 2020. Workshop Report on Biodiversity and Pandemics of the Intergovernmental Platform on Biodiversity and Ecosystem Services.https://ipbes.net/sites/default/files/2020-12/IPBES%20Workshop%20on%20Biodiversity%20and%20Pandemics%20Report_0.pdf [DOI] [Google Scholar]
- 3.Huong N.Q., Nga N.T.T., Long N.V., Luu B.D., Latinne A., Pruvot M., et al. Coronavirus testing indicates transmission risk increases along wildlife supply chains for human consumption in Viet Nam, 2013-2014. PLoS One. 2020;15(8) doi: 10.1371/journal.pone.0237129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carrique-Mas J.J., Bryant J.E. A review of foodborne bacterial and parasitic zoonoses in Vietnam. EcoHealth. 2013;10:465–489. doi: 10.1007/s10393-013-0884-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trang do T., Siembieda J., Huong N.T., Hung P., Ky V.D., Bandyopahyay S., et al. Prioritization of zoonotic diseases of public health significance in Vietnam. J. Infect. Dev. Ctries. 2015;9(12):1315–1322. doi: 10.3855/jidc.6582. [DOI] [PubMed] [Google Scholar]
- 6.Ministry of Health–Ministry of Agriculture and Rural Development . Joint Circular: Guidelines for coordinated prevention and control of zoonotic diseases. 2013. Ref. No. 16/2013/TTLT-BYT-BNN&PTNT. Hanoi, Vietnam, May 27. [Google Scholar]
- 7.https://www.cdc.gov/onehealth/basics/index.html
- 8.Ministry of Health . Guidance on reporting and declaration of infectious diseases and epidemics. 2015. Ref. No. 54/2015/TT-BYT. Hanoi, Vietnam, December 28.http://vbpl.yte.gov.vn/van-ban-phap-luat/thong-tu-542015tt-byt.6.1508.html [Google Scholar]
- 9.Ministry of Agriculture & Rural Development Ref. No. 07/2016/TT-BNNPTNT. Regulations on prevention, control of epidemics on terrestrial animals. http://www.cucthuy.gov.vn/vanban/Pages/thong-tu-07-2016-tt-bnnptnt.aspx
- 10.MSD and the MSD Veterinary Manual Zoonotic Diseases. 2021. https://www.msdvetmanual.com/public-health/zoonoses/zoonotic-diseases#v3357819 Accessed on 29 Jan.
- 11.https://www.cdc.gov/healthypets/diseases/index.html
- 12.https://www.gov.uk/government/publications/list-of-zoonotic-diseases/list-of-zoonotic-diseases
- 13.Kobo-tool Box United Nations Office for the Coordination of Humanitarian Affairs. https://www.humanitarianresponse.info/applications/kobotoolbox
- 14.Wang X., Rainey J.J., Goryoka G.W., Liang Z., Wu S., Wen L., et al. Using a one health approach to prioritize zoonotic diseases in China, 2019. PLoS One. 2021;16(11) doi: 10.1371/journal.pone.0259706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mersha T.T., Mekonnen Wolde B., Shumuye N.A., Hailu A.B., Mohammed A.H., Redda Y.T., et al. Prioritization of neglected tropical zoonotic diseases: a one health perspective from Tigray region, Northern Ethiopia. PLoS One. 2021;16(7) doi: 10.1371/journal.pone.0254071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ihekweazu C., Michael C.A., Nguku P.M., Waziri N.E., Habib A.G., Muturi M., et al. Prioritization of zoonotic diseases of public health significance in Nigeria using the one-health approach. One Health. 2021;13 doi: 10.1016/j.onehlt.2021.100257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yasobant S., Saxena D., Bruchhausen W., Memon F.Z., Falkenberg T. Multi-sectoral prioritization of zoonotic diseases: one health perspective from Ahmedabad, India. PLoS One. 2019;14(7) doi: 10.1371/journal.pone.0220152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sekamatte M., Krishnasamy V., Bulage L., Kihembo C., Nantima N., Monje F., et al. Multisectoral prioritization of zoonotic diseases in Uganda, 2017: a One Health perspective. PLoS One. 2018;13(5) doi: 10.1371/journal.pone.0196799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kadohira M., Hill G., Yoshizaki R., Ota S., Yoshikawa Y. Stakeholder prioritization of zoonoses in Japan with analytic hierarchy process method. Epidemiol. Infect. 2015;143(7):1477–1485. doi: 10.1017/S0950268814002246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munyua P., Bitek A., Osoro E., Pieracci E.G., Muema J., Mwatondo A., et al. Prioritization of zoonotic diseases in Kenya, 2015. PLoS One. 2016;11(8) doi: 10.1371/journal.pone.0161576. [DOI] [PMC free article] [PubMed] [Google Scholar]