Abstract
Introduction
Arteriolar microangiopathic (MA) lesions are independent risk factors for IgA nephropathy (IgAN) patient prognosis, and the underlying mechanism remains to be elucidated. The complement plays an important role in IgAN and thrombotic microangiopathy; however, its role in MA lesions in IgAN remains unclear.
Methods
Immunohistochemistry was performed to detect arteriolar complement deposition. Enzyme-linked immunosorbent assay (ELISA) and whole-exome sequencing were performed to explore possible mechanism.
Results
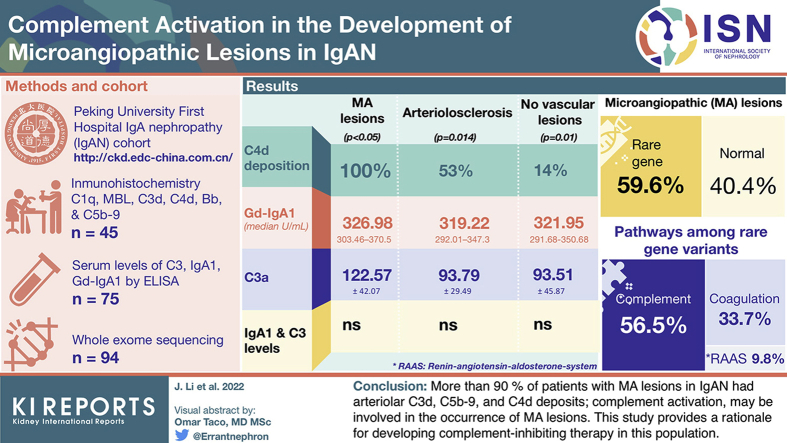
In this study, we found that patients with IgAN with MA lesions have more complement deposition, especially C4d, on renal arterioles than those in patients with arteriolosclerosis (AS) and patients with no vascular lesions (100% vs. 53% vs. 14%, P < 0.05). Furthermore, our large prospective cohort demonstrated that patients with IgAN with MA lesions had higher levels of Gd-IgA1 (median: 326.98 U/ml, interquartile range 303.46–370.5 vs. 319.22, 292.01–347.3 vs. 321.95, 291.68–350.68, P = 0.014) and C3a (122.57 ± 42.07 vs. 93.79 ± 29.49 vs. 93.51 ± 45.87 ng/ml, P = 0.01) than those in patients with AS and those with no vascular lesions. However, serum IgA1 and C3 levels were not significantly different. Finally, through whole-exome sequencing, we found that nearly half of the patients with MA lesions had rare genetic variations in complement-related genes.
Conclusion
Our results indicate that the complement is involved in the development of MA lesions in IgAN, which might be associated with the circulating complex containing Gd-IgA1.
Keywords: complement activation, IgA nephropathy, microangiopathic lesions
Graphical abstract
IgAN is the most common primary glomerular disease worldwide.1 Nearly 20% to 40% of patients progress to end-stage renal disease.2 Its pathologic manifestations are mainly expressed as IgA deposition in the mesangial area, with >90% of patients expressing codominant C3 deposition. Pathologic lesions include mesangial hypercellularity (M), endocapillary hypercellularity (E), segmental glomerulosclerosis (S), interstitial fibrosis/tubular atrophy (T), and crescentic lesions (C) associated with the progression of kidney disease. These parameters are included in the latest version of the Oxford classification for IgAN.3
Intrarenal arterial lesions are common in patients with IgAN. Some recent studies suggest that MA lesions, a type of arterial lesion, are strongly associated with kidney failure.4, 5, 6, 7, 8 Our earlier study demonstrated9 that approximately 20% of patients with IgAN had MA lesions. Until recently, the mechanism of the MA lesions was still unclear, and specific therapy for this lesion was lacking. Although MA lesions are often associated with severe hypertension, a cause-and-effect relationship has not been established and several studies have demonstrated that not all patients with MA show hypertension.9, 10, 11 Previous studies reported that 15.8% and 20% of patients with MA lesions had no history of hypertension.9,11 A recent study suggested that some so-called malignant hypertension-related thrombotic MA because of complement activation is not a result of hypertension.10 It was also suggested by another study that arteriolar C4d deposits were an independent risk factor for poor prognosis of IgAN.12 Another study found that complement activation is involved in the development of MA in patients with IgAN.13 These data suggest that other factors might contribute to the development of MA lesions in IgAN, especially complement activation.
The aim of this study was to investigate the activation of the complement in the development of MA lesions in IgAN and to further explore its possible mechanism.
Methods
Patients
Patients with IgAN were selected from the Peking University First Hospital IgAN cohort (http://ckd.edc-china.com.cn/); patients lacking plasma samples and renal biopsy specimen were excluded. For immunohistochemical staining, the renal biopsy tissues were obtained from 45 patients with IgAN, which comprised 15 patients with MA lesions, 15 with arteriosclerosis/AS lesions (AS group), and 15 without any vascular lesions (NO group). To detect C3a levels, frozen serum that has never been thawed was obtained from 75 patients; the number of patients in each group was 25. These 3 groups of patients were comparable in age, sex, proteinuria, estimated glomerular filtration rate, and MESTC scores. All these patients had >8 glomeruli and >6 vessels in their biopsy sections. Renal biopsy specimens from minimal change disease were used as negative control.
For whole-exome sequencing, 94 patients with IgAN with MA lesions were selected from our cohort.
Every patient signed the informed consent when entering the IgAN cohort. This study has been approved by the Ethics Committee of the institution.
Pathologic Evaluation
Kidney biopsy specimens were routinely stained with Masson’s trichrome, periodic acid–Schiff, hematoxylin and eosin, and silver using standard protocols. All specimens were graded by 2 independent pathologists who were blinded to all information. They re-evaluated renal vascular lesions, including MA lesions and AS. As described in previous study,9 MA lesions include endothelial cell swelling and subintimal edema, arteriolar thrombosis, and/or fibrinoid necrosis. Lesions with arterial “onion-skin” are also classified as MA lesions. AS lesions include arterial intimal fibrosis and arteriolar hyalinosis (Supplementary Figure S1).
Detection of Complement Activation by Immunohistochemistry
Immunohistochemistry was performed to detect C1q, MBL, C3d, C4d, Bb, and C5b-9. All tissues were 10% formalin fixed, paraffin embedded, and cut into 2-μm thick. First, after deparaffinization in xylene and rehydration in different concentrations of alcohol, sections were treated by appropriate predetermined antigen retrieval methods, including pepsin incubation, heating in an autoclave containing EDTA solutions, and combination of both. Then, sections were treated in 3% hydrogen peroxide for 10 to 15 minutes at room temperature in the dark and were placed in 3% bovine serum albumin. The liquid was shaken off directly and then treated with the primary antibody for 1 hour at 37 °C. After washing, the sections were incubated with the horseradish peroxidase–conjugated secondary antibody for 20 minutes at room temperature. Then, the sections were stained with diaminobenzidine for proper time, and after washing, the sections were counterstained with Mayer’s hematoxylin. Finally, the sections were dehydrated and cleared in alcohol and xylene and coverslipped with Permount. Pictures were taken with an optical microscope (Nikon, Japan).
Semiquantitative Scoring
Glomerular positivity was defined as the positive staining of nonsclerosing glomeruli. Positive arterioles were defined as circumferential staining along the vessel lumina, and positivity only present along the elastic lamina was excluded. In our study, we scored the arterioles that were stained, but not the glomeruli, because we mainly focused on arterioles.
For a better assessment of complement deposition, we used a scoring system that involved the combination of the proportion of stained vessels and the intensity of the stain. Using this methodology, some laboratories find that the results are highly reproducible and correlate well with the old biochemical assays.14 The intensity of staining was evaluated at 400× magnification in a high-power field and scored on a scale of 0 to 3: 0, no staining; 1, mild staining; 2, moderate staining; and 3, high staining. The score of proportion staining was as follows: 0, there are no positive vessels; 1, >0 and <25% of vessels are positive; 2, >25% and <50% of vessels are positive; and 3, >50% of the vessels are positive. The final result is the sum of the 2 values, ranging from 0 to 6.
Detection of Serum Levels of C3a by ELISA
Circulating C3a was detected by an ELISA kit (Quidel, San Diego, CA). All operating procedures were performed in strict accordance with the manufacturer’s instructions.
Detection of Serum Levels of IgA1, Gd-IgA1 by ELISA
Circulating IgA1 and Gd-IgA1 were detected by ELISA as previously described.15 Briefly, high-binding MaxiSorp 96-well plates (Nalge Nunc, Rochester, NY) were coated with F(ab’) 2 fragment of goat antihuman IgA (Jackson Immuno-Research Labs, West Grove, PA). After overnight incubation at 4 °C, the coated plates were washed and blocked with 1% bovine serum albumin (Sigma Chemical Company, St Louis, MO). Then, diluted serum samples and standards were added. After incubating for 1 hour, the level of IgA1 was determined by incubation with horseradish peroxidase–labeled mouse antihuman IgA1 antibody. As for Gd-IgA1, samples were treated with sialidase A and biotin-labeled helix aspersa agglutinin after incubating. After another incubation, the plates were further incubated with horseradish peroxidase-ExtrAvidin (Sigma). The plates were then developed with the peroxidase chromogenic substrate o-phenylenediamine-hydrogen peroxide (Sigma). The color reaction was stopped with 1 M sulfuric acid, and the absorbance was measured at 490 nm with an EL312 Bio-Kinetics microplate reader (Bio-Tek Instruments Inc., Winooski, VT).
Genetic Testing
Genomic DNA was extracted from the peripheral blood of the patients. Exome capture was performed using the IDT XGen Exome Research Panel version 1.0 capture kits (Coralville, IA). Sequencing was performed on the Illumina HiSeq2500 and NovaSeq platforms (San Diego, CA). Sequencing data were aligned to the reference human genome (hg19) using the Burrows-Wheeler Aligner. The Genome Analysis Toolkit was used to identify the variants. The ANNOVAR software was used to annotate the detected variations. Then, after removing variants with low quality and a minor allele frequency higher than 0.01% in the Genome Aggregation Database (gnomAD, https://gnomad.broadinstitute.org), variants were filtered by leaving coding, nonsense mutations, frame shifts (indels), and splicing modifying changes. Using in silico analyses, including PolyPhen-2, SIFT, PROVEAN, and MutationTaster, variants with a high pathogenicity prediction were defined as predicted pathogenic mutations. A pathogenicity score of 2 was considered to be pathogenic. The genes involved in complement, coagulation, and renin-angiotensin system were analyzed.
Statistics
Continuous variables are expressed as the mean ± SD (normally distributed variables), or expressed as the median with 25th and 75th percentiles (non-normally distributed variables) for data distribution. In addition, categorical variables were summarized as frequencies with percentages. Comparison between 2 groups was determined by an independent sample t test or Mann-Whitney U test. Comparison between 3 groups was determined by one-way analysis of variance or the Kruskal–Wallis H test. Comparison of categorical variables was performed by χ2 test.
Results
Clinical and Pathologic Characteristics of Patients
The general clinicopathologic information of the patient is listed in Supplementary Table S1. Patients with MA lesions showed more severe clinical parameters, including higher blood pressure, urine proteinuria, and lower estimated glomerular filtration rate.
For 45 patients undergoing immunohistochemical staining, their clinical data are shown in Table 1. For 75 patients who were included for circulating C3a measurement, their clinical data are shown in Table 2.
Table 1.
Clinicopathologic features of 3 groups performed immunohistochemistry
| Characteristics | Microangiopathic lesions n = 15 | Simple arterio-/arteriolosclerosis n = 15 | No vascular lesion n = 15 | P | Pa | Pb | Pc |
|---|---|---|---|---|---|---|---|
| Sex, male (n, %) | 10 (66.7%) | 10 (66.7%) | 11 (77.3%) | 1 | NS | NS | NS |
| Age (yr) | 29 (26–35) | 42 (31–47) | 27 (23–43) | 0.062 | NS | NS | NS |
| Systolic BP (mm Hg) | 133.6 ± 9.434 | 123.07 ± 16.329 | 128.6 ± 16.741 | 0.27 | 0.393 | 0.44 | 0.108 |
| Diastolic BP (mm Hg) | 88.6 ± 15.188 | 80.47 ± 9.848 | 82.87 ± 12.727 | 0.213 | 0.61 | 0.226 | 0.089 |
| Proteinuria (g/24 h) | 3.2 (2.4–4.6) | 2.2 (1.2–3.7) | 1.6 (0.7–5.0) | 0.53 | NS | NS | NS |
| Scr (μmol/l) | 123 (111.7–198) | 115.5 (71.7–147.9) | 132 (70.8–150.8) | 0.313 | NS | NS | NS |
| eGFR (ml/min per 1.73 m2) | 55.12 ± 25.5 | 72.3 ± 32.8 | 70.9 ± 36.2 | 0.276 | 0.905 | 0.182 | 0.154 |
| Hemoglobin (g/l) | 135.5 ± 21.5 | 130.2 ± 21.4 | 133.9 ± 20.8 | 0.788 | 0.639 | 0.837 | 0.505 |
| Platelet count (109/l) | 255.1 ± 58.1 | 221.7 ± 47.2 | 267.6 ± 68.1 | 0.098 | 0.038 | 0.567 | 0.132 |
| Albumin (g/l) | 37.3 ± 5.6 | 35.8 ± 7.6 | 33.52 ± 7.3 | 0.316 | 0.385 | 0.133 | 0.533 |
| Oxford classification (n, %) | |||||||
| M1 | 14 (93.3%) | 12 (80.0%) | 14 (93.3%) | 0.415 | NS | NS | NS |
| E1 | 5 (33.5%) | 3 (20.0%) | 8 (53.3%) | 0.165 | NS | NS | NS |
| S1 | 12 (80%) | 9 (60.0%) | 14 (93.3%) | 0.092 | NS | NS | NS |
| T1/T2 | 6 (40%)/6 (40%) | 5 (33.3%)/3 (20.0%) | 9 (60.0%)/2 (13.3%) | 0.217 | NS | NS | NS |
| C1/C2 | 7 (46.7%)/2 (13.3%) | 8 (53.3%)/3 (20.0%) | 8 (53.3%)/6 (40%) | 0.061 | NS | NS | NS |
Unless otherwise indicated, the values represent n (%), the mean ± SD, or the median (25th–75th centiles).
BP, blood pressure; C, crescent; E, endocapillary hypercellularity; eGFR, estimated glomerular filtration rate; M, mesangial hypercellularity; NS, not significant; S, segmental glomerulosclerosis; Scr, serum creatinine; T, interstitial fibrosis and tubular atrophy.
P values between groups with no vascular lesions and simple arterio-/arteriolosclerosis.
P values between groups with no vascular lesions and microangiopathic lesions.
P values between groups with simple arterio-/arteriolosclerosis and microangiopathic lesions.
Table 2.
Clinicopathologic features of 3 groups with nonvascular lesions, simple arteriosclerosis/arteriolosclerosis, and microangiopathic lesions
| Characteristics | Microangiopathic lesions n = 25 | Simple arterio-/arteriolosclerosis n = 25 | No vascular lesion n = 25 | P | Pa | Pb | Pc |
|---|---|---|---|---|---|---|---|
| Sex, male (n, %) | 18 (72%) | 14 (56%) | 18 (72%) | 0.383 | NS | NS | NS |
| Age (yr) | 36 (29.25–44.25) | 36 (29.5–50) | 35 (26–46.5) | 0.54 | NS | NS | NS |
| Systolic BP (mm Hg) | 137 ± 15.155 | 131.76 ± 15.696 | 130.52 ± 15.18 | 0.291 | 0.776 | 0.14 | 0.231 |
| Diastolic BP (mm Hg) | 86.32 ± 13.588 | 83.4 ± 12.007 | 81.08 ± 11.814 | 0.337 | 0.514 | 0.143 | 0.411 |
| Proteinuria (g/24 h) | 3.22 (2.05–6.34) | 2.54 (1.4–5.26) | 2.2 (1.01–4.05) | 0.188 | NS | NS | NS |
| Scr (μmol/l) | 199.8 (176.1–280.8) | 191.3 (118.25–286.15) | 162.56 (97.95–249.56) | 0.149 | NS | NS | NS |
| eGFR (ml/min per 1.73 m2) | 32.8495 ± 15.8837 | 41.869 ± 28.784 | 47.295 ± 25.843 | 0.109 | NS | NS | NS |
| Hemoglobin (g/l) | 119.4 ± 22.46 | 120.79 ± 19.713 | 129.67 ± 20.08 | 0.184 | 0.144 | 0.089 | 0.816 |
| Platelet count (109/l) | 238.44 ± 55.66 | 225.79 ± 62.66 | 245.13 ± 59.601 | 0.521 | 0.263 | 0.695 | 0.458 |
| Albumin (g/l) | 35.66 ± 5.79 | 34.844 ± 9.1 | 38.113 ± 5.59 | 0.247 | 0.108 | 0.226 | 0.683 |
| Oxford classification (n, %) | |||||||
| M1 | 19 (76%) | 19 (76%) | 17 (68%) | 0.761 | NS | NS | NS |
| E1 | 10 (40%) | 8 (32%) | 12 (48%) | 0.513 | NS | NS | NS |
| S1 | 24 (96%) | 19 (76%) | 20 (80%) | 0.128 | NS | NS | NS |
| T1/T2 | 13 (52%)/10 (40%) | 12 (48%)/7 (28%) | 11 (44%)/2 (8%) | 0.002 | NS | NS | NS |
| C1/C2 | 19 (76%)/1 (4%) | 11(44%)/5 (20.0%) | 18 (72%)/3 (12%) | 0.677 | NS | NS | NS |
BP, blood pressure; C, crescent; E, endocapillary hypercellularity; eGFR, estimated glomerular filtration rate; M, mesangial hypercellularity; NS, not significant; S, segmental glomerulosclerosis; Scr, serum creatinine; T, interstitial fibrosis and tubular atrophy.
P values between groups with no vascular lesions and simple arterio-/arteriolosclerosis.
P values between groups with no vascular lesions and microangiopathic lesions.
P values between groups with no vascular lesions and microangiopathic lesions.
There were 94 patients with MA lesions selected for whole-exome sequencing, and the clinical and pathologic characteristics are listed in Supplementary Table S2.
Deposition of Complement Components
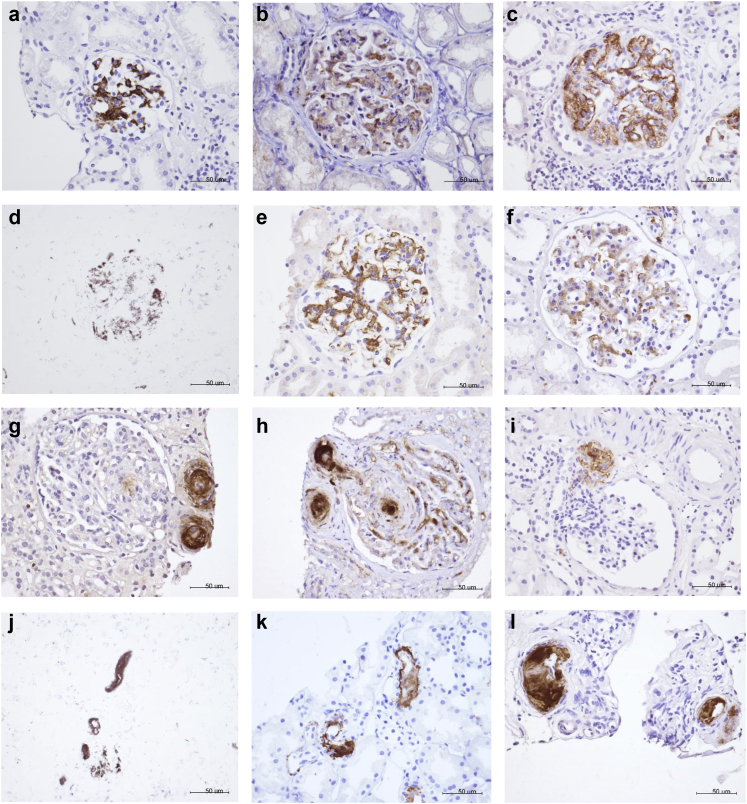
The complements are deposited in both the glomeruli and arterioles, as shown in Figure 1. In the glomerulus, the complement is generally manifested as mesangial deposition or concomitantly in the glomerular capillary walls (Figure 1a-f). In the arterioles, the complement is nearly confined to endothelial cells (Figure 1g-l).
Figure 1.
Typical examples of complement deposits in the glomeruli (a–f) and the arterioles (g–i). a and g represent the deposition of C1q. b and h represent the deposition of MBL. c and i represent the deposition of C4d. d and j represent the deposition of Bb. e and k represent the deposition of C3d. f and l represent the deposition of C5b-9 (MAC). a–l: original magnification, ×400.
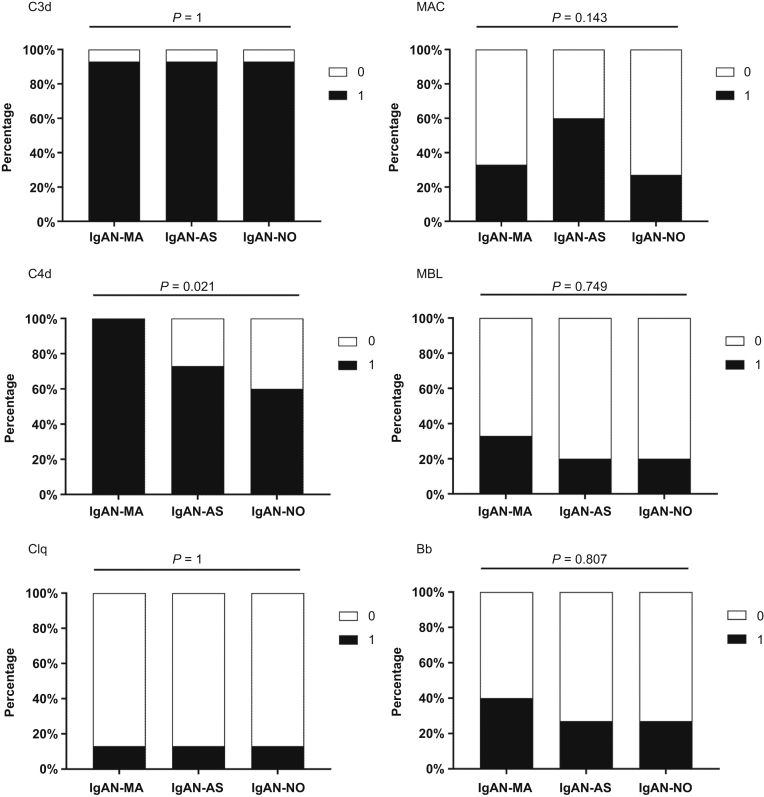
Regarding complement activation in the glomeruli, the proportion of C4d deposition in the MA group was much higher than that in the AS and NO groups (100% vs. 73% vs. 60%, P = 0.021), but there were no differences among the 3 groups with regard to C3d, C5b-9 (MAC), C1q, MBL, and Bb (Figure 2).
Figure 2.
Differences of complement deposits in glomeruli between IgAN-MA (n = 15), IgAN-AS (n = 15), and IgAN-NO (n = 15). All data are expressed as n (%), analyzed by χ2 test. IgAN, IgA nephropathy; IgAN-MA, patients with IgAN with microangiopathic lesions; IgAN-AS, patients with IgAN with arteriosclerosis/arteriolosclerosis lesions; IgAN-NO, patients with IgAN without vascular lesions.
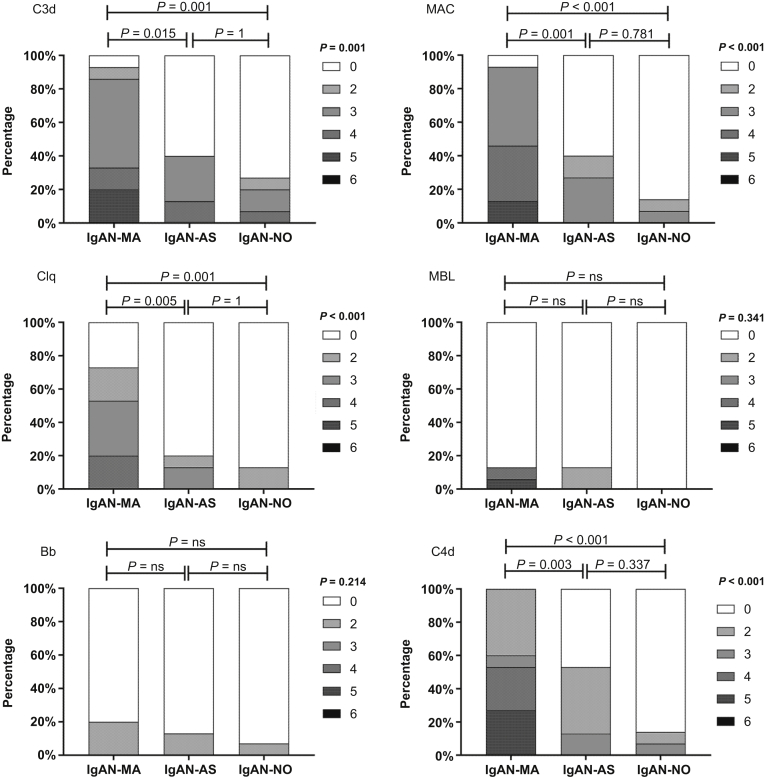
The C3d and C5b-9 (MAC) deposits represent complement activation. In the arterioles, 14 of 15 patients with MA lesions had deposition of C3d and C5b-9 (both 93%), both of which were higher than that in patients with AS lesions (both 40%) and without vascular lesions (27% and 14%, respectively). As shown in Figure 3, the C3d and C5b-9 deposition scores were much higher in patients with MA lesions than in those with AS lesions (P = 0.015 and 0.001, respectively) or without vascular lesions (both P < 0.001).
Figure 3.
Differences of complement deposition score in arterioles between IgAN-MA (n = 15), IgAN-AS (n = 15), and IgAN-NO (n = 15). All data are expressed as n (%). The scores were analyzed using the Kruskal–Wallis test. IgAN, IgA nephropathy; IgAN-MA, patients with IgAN with microangiopathic lesions; IgAN-AS, patients with IgAN with arteriosclerosis/arteriolosclerosis lesions; IgAN-NO, patients with IgAN without vascular lesions; ns, not significant.
The deposition of C4d, representing lectin pathway activation in IgAN, was more prevalent in the MA group than in the other 2 groups, and the positive rates of C4d were 100%, 53%, and 14%, respectively. The deposition score of C4d in the MA group was higher than that in the other 2 groups (P < 0.001). MBL was detected in 2 (13%), 2 (13%), and 0 (0%) of 15 patients, and Bb was detected in 3 (20%), 2 (13%), and 1 (7%) of 15 patients, respectively. The positive rates and deposition scores of MBL and Bb were not significantly different among the 3 groups (P = 0.341, P = 0.214). Interestingly, we noticed that the proportions of C1q deposition in the 3 groups were 73%, 20%, and 13%, respectively (P < 0.001).
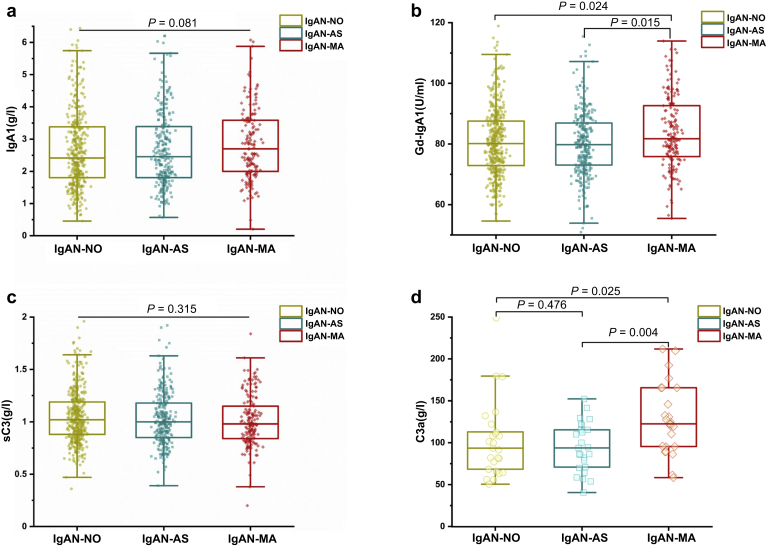
Plasma Levels of IgA1, Gd-IgA1, C3, and C3a
As shown in Figure 4a, there was no difference in the plasma IgA1 levels (median 2.7 mg/ml, interquartile range 2.0–3.58 vs. 2.47, 1.81–3.43 vs. 2.54, 1.81–3.46; P = 0.227) among the 3 groups of patients. However, the levels of Gd-IgA1 in patients with MA lesions were much higher than those in the other 2 groups of patients (median 326.98 U/ml, interquartile range 303.46–370.5 vs. 319.22, 292.01–347.3 vs. 321.95, 291.68–350.68; P = 0.014; Figure 4b).
Figure 4.
Serum levels of IgA1, Gd-IgA1, sC3, and C3a in patients with IgAN of 3 groups. a–d: IgA1, Gd-IgA1, sC3, and C3a levels, respectively. IgAN, IgA nephropathy; IgAN-MA, patients with IgAN with microangiopathic lesions; IgAN-AS, patients with IgAN with arteriosclerosis/arteriolosclerosis lesions; IgAN-NO, patients with IgAN without vascular lesions.
Plasma C3 levels of patients with IgAN with MA lesions showed no difference as compared with the other IgAN (median 0.98 g/l, interquartile range 0.84–1.15 vs. 1.0, 0.85–1.18 vs. 1.02. 0.88–1.2; P = 0.2; Figure 4c). However, the levels of plasma C3a levels in patients with IgAN with MA lesions were much higher than those in the other 2 groups of patients (122.57 ± 42.07 vs. 93.79 ± 29.49 vs. 93.51 ± 45.87 ng/ml; P = 0.01; Figure 4d).
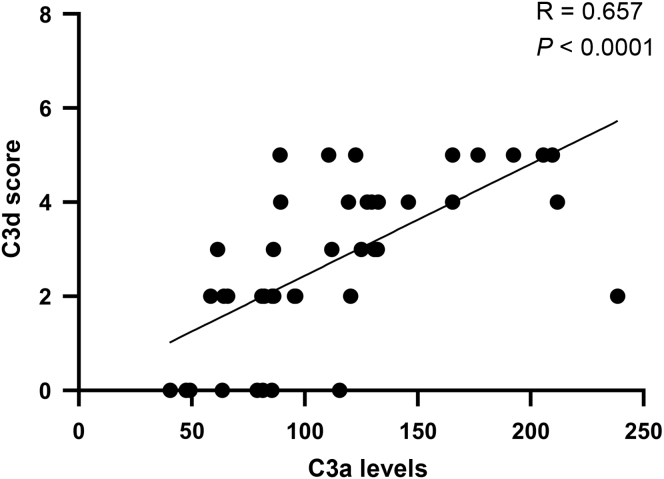
Association of the Levels of Plasma C3a and Arteriolar C3d Deposition
Among the 120 patients with IgAN, 45 patients with both histologic sections and qualified plasma were selected for correlation analysis, including 28 patients with MA lesions, 9 patients with AS lesions, and 8 patients without vascular lesions. As shown in Figure 5, we found that the plasma levels of C3a showed a linear association with the arteriolar C3d deposition (r = 0.657; P < 0.0001). This result indicated that cases with more complement deposition at arterioles have a high probability of complement overactivation in plasma.
Figure 5.
The plasma levels of C3a are in association with the arteriolar C3d deposition.
Rare Variants of Corresponding Genes
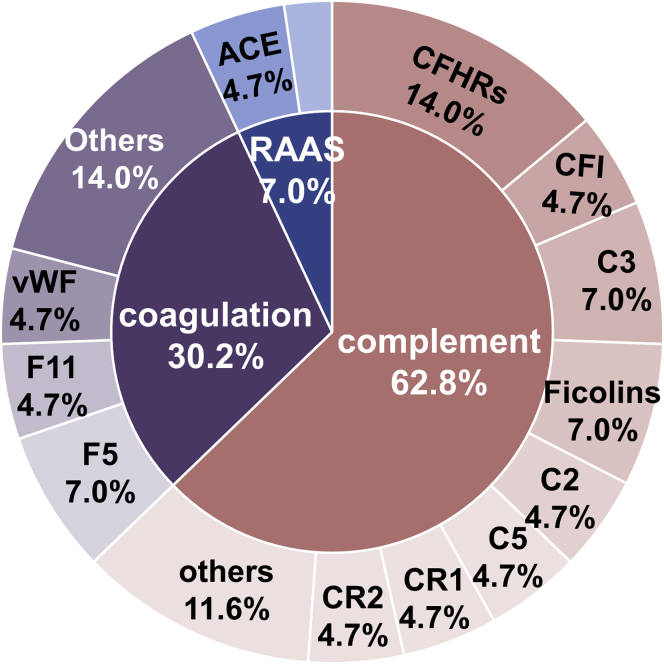
Overall, 92 rare gene variants were detected in 56 patients (59.6%) with MA lesions. There were 40 patients (42.6%) who had gene variants in the complement pathway, 23 patients (24.5%) had variants in the coagulation pathway, and 5 patients (5.3%) had variants in the renin-angiotensin-aldosterone system. In addition, 10 patients had gene variants involved in 2 pathways, and only 1 patient had variants in 3 pathways.
Among all rare gene variants, 52 (56.5%) belonged to the complement pathway, 31 (33.7%) belonged to the coagulation pathway, and 9 (9.8%) belonged to the renin-angiotensin-aldosterone system. On the basis of in silico analysis, the possible pathogenicity of the 92 mutations was assessed, and by combining 4 computational prediction methods, 35 variants were predicted to be potentially pathogenic (Supplementary Table S3).
Except these 92 variants of which the minor allele frequency is lower than 0.01%, there are another 14 reported gene variants of which the minor allele frequency is between 1% and 0.1%, and 8 of them were reported as pathogenic or potentially pathogenic (Supplementary Table S4). Overall, 43 gene variants (35 predicted and 8 potentially pathogenic variants) were identified in 35 patients, and of these variants, 27 (62.8%) were found in the complement pathway genes, 13 (30.2%) were found in coagulation pathway genes, and only 3 were associated with the renin-angiotensin-aldosterone system (Figure 6). The greatest number of potentially pathogenic variants was found in CFHRs (6 variants in 7 patients; 3 were CFHR5, 2 were CFHR3, and 1 CFHR2). The second highest number of potentially pathogenic variants was found in C3 and F5. Overall, the most potentially pathogenic variants are located in the complement system pathway, and the coagulation pathway also makes an important contribution.
Figure 6.
Distribution of reported and predicted potentially pathogenic mutations. A detailed description is listed in Supplementary Tables S3 and S4.
Discussion
In this study, we found that patients with IgAN with MA lesions had more complement activation product deposits on arterioles, with >90% of patients having C3d and C5b-9 positivity. This suggests that overcomplement activation is involved in the development of MA lesions in IgAN. Furthermore, data showed that there were more C4d deposits, but there was no difference in Bb deposits between patients with MA lesions and those with atherosclerotic or no vascular lesions. Our results suggest that overactivation of the complement contributes to the development of MA lesions in IgAN. Our findings provide a new treatment strategy for this population in the future.
Intrarenal arterial lesions are common in IgAN and are usually thought to be a consequence of hypertension; however, a causal relationship has not yet been established. Complement activation has been confirmed to be involved in the development and progression of IgAN. Furthermore, Chua et al.13 reported that MA lesions in IgAN or IgA vasculitis with nephritis were associated with C4d and C5b-9 deposits, suggesting complement activation might be involved in arteriole MA. Faria et al.12 further reported that arteriole C4d deposits are associated with a high risk of kidney disease progression. In this study, we systematically investigated the association between complement activation and vascular lesions in patients with IgAN. There was more complement activation in the arterioles of patients with MA than in the other 2 groups. We found that 93% and 100% of patients with MA had arteriole C5b-9 and C4d deposits, respectively. Consistent with the arteriole staining results, all patients with MA had glomerular C4d deposition. These results suggest that complement activation is involved in the development of MA. However, the study did not include patients with MA lesions owing to other disease and lack of independent cohort validation to provide more objective results.
Glomerular C4d deposits are an independent risk factor for poor prognosis of IgAN. We found that more than two-thirds of Chinese patients had glomerular C4d deposits, which was much higher than that in the Caucasian population (20%–43%).16 Furthermore, in our cohort, <10% of patients had C1q deposits in the glomeruli. These data suggest that complement activation of the lectin pathway involves the development of IgAN in the Chinese population. This difference may partially explain why the Asian-Pacific population showed a high risk of progression in IgAN. Interestingly, in this study, we found that the positive rate of arteriole C1q in patients with MA lesions was 73%, which was similar to the results of The Netherlands cohort.13 In addition, C1q deposits were also observed to be as high as 70% in atypical hemolytic uremic syndrome.17 These results suggest widespread C1q deposition in arterioles in patients with MA or other thrombotic microangiopathy. However, different from the French cohort in which arteriolar IgM deposition is present in 77.5% of patients with primary thrombotic microangiopathy lesions,17 most of patients with MA with C1q-positive arterioles did not have IgG or IgM deposition in the IgAN cohort (data not shown). Therefore, the mechanism of C1q deposition in MA lesions of IgAN is not very clear. In recent years, some studies have suggested that C1q does not play a role in complement activation sometimes but plays an important role in vascular repair and acts as a unique angiogenic factor.18 The C1q may also represent a secondary deposition because of MA lesions. Finally, our group found that 63% of patients with IgAN have C4d deposition but only 40% have MBL deposition.19 Collectin-11 and L-ficolin were also involved in the activation of the lectin pathway. Thus, arteriolar C4d deposits might represent the lectin pathway complement activation. Overall, our study suggests that complement activation is involved in the MA lesions in IgAN; however, the exact pathway still needs further study.
Our study suggests that complement activation contributes to both more severe glomerular lesions and vascular lesions. Aberrant glycosylation of circulating polymeric IgA1 may be involved in lectin complement activation and abnormal coagulation, which in turn leads to MA lesions. In this study, we preliminarily analyzed rare gene variants in the complement and coagulation pathways and renin-angiotensin-aldosterone system in MA lesions. Our results showed that more than half of the patients had rare gene variants, and 37.3% of the patients were predicted or reported to have potentially pathogenic variants. Of all potentially pathogenic variants, variants in the complement pathway were dominant, with an alternative pathway accounting for 27.9% and lectin pathway accounting for 14.0%. However, in this study, we only screened for possible pathogenic variants and did not verify the functional effect of them. This is one of the limitations of this study. In our prior study, several variants of CFHR5 including the variants detected in this study were found having significantly higher C3b binding capacity which can indirectly promote complement activation.20 Another limitation of this study was lacking genetic analysis of controls in patients without MA lesions. In this study, we try to evaluate whether there were rare gene variants of the complement system in the patients with MA lesions as other similar studies in atypical hemolytic uremic syndrome.21,22 Further analysis is still needed in a control cohort without MA lesions.
Prior studies, including our cohort study, consistently demonstrated that arteriolar MA was an independent risk factor for kidney failure9; however, specific therapies for this disease are still lacking. Our study provides further evidence that complement activation is involved in the development of vascular lesions. This is particularly interesting, given recent advances in complement-inhibiting therapeutics. As a human monoclonal antibody against the complement component mannan-associated lectin-binding serine protease 2,23 interim analysis suggests that narsoplimab treatment may lead to a decrease in proteinuria and increase the stability of estimated glomerular filtration rate in high-risk patients with advanced IgAN. The study is of great clinical significance. Future studies are needed to confirm the role of narsoplimab in patients with IgAN with MA.
In conclusion, our study demonstrated that >90% of patients with MA lesions in IgAN had arteriolar C3d, C5b-9, and C4d deposits. These results suggest that complement activation may be involved in the occurrence of MA lesions. This study provides a rationale for developing complement-inhibiting therapy in this population.
Disclosure
All the authors declared no competing interests.
Acknowledgments
The authors are grateful to all the patients for their participation in this study. This work was supported by the National Natural Science Foundation of China (81925006, 82070733), the National Key Research and Development Program of China (2018YFC1314004), the Capital Health Development Research Project of China (2018-2-4073), the Capital of Clinical Characteristics, the Applied Research Fund (Z161100000516005), Grants from the Science and Technology Project of Beijing, China (D18110000011803), and CAMS Innovation Fund for Medical Sciences (2019-12M-5-046).
Author Contributions
JYL designed the study, performed the experiments, analyzed the data, and wrote the manuscript. LG contributed to the patient selection. SS contributed to the interpretation of the results with XZ, LZ, JL, and HZ. JL also designed the study and provided critical revisions to the manuscript.
Footnotes
Figure S1. Typical examples of MA lesions and arteriolosclerosis in IgA nephropathy.
Table S1. Clinicopathologic features of groups with nonvascular lesions, simple arterio-/arteriolosclerosis, and microangiopathic lesions.
Table S2. Clinicopathologic features of 94 patients.
Table S3. Predicted potentially pathogenic mutations.
Table S4. Reported potentially pathogenic mutations.
Supplementary Material
Figure S1. Typical examples of MA lesions and arteriolosclerosis in IgA nephropathy.
Table S1. Clinicopathologic features of groups with nonvascular lesions, simple arterio-/arteriolosclerosis, and microangiopathic lesions.
Table S2. Clinicopathologic features of 94 patients.
Table S3. Predicted potentially pathogenic mutations.
Table S4. Reported potentially pathogenic mutations.
References
- 1.Wyatt R.J., Julian B.A. IgA nephropathy. N Engl J Med. 2013;368:2402–2414. doi: 10.1056/NEJMra1206793. [DOI] [PubMed] [Google Scholar]
- 2.Berthoux F.C., Mohey H., Afiani A. Natural history of primary IgA nephropathy. Semin Nephrol. 2008;28:4–9. doi: 10.1016/j.semnephrol.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Trimarchi H., Barratt J., Cattran D.C., et al. Oxford Classification of IgA nephropathy 2016: an update from the IgA Nephropathy Classification Working Group. Kidney Int. 2017;91:1014–1021. doi: 10.1016/j.kint.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Coppo R., Troyanov S., Bellur S., et al. Validation of the Oxford classification of IgA nephropathy in cohorts with different presentations and treatments. Kidney Int. 2014;86:828–836. doi: 10.1038/ki.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeng C.H., Le W., Ni Z., et al. A multicenter application and evaluation of the oxford classification of IgA nephropathy in adult Chinese patients. Am J Kidney Dis. 2012;60:812–820. doi: 10.1053/j.ajkd.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 6.Shi S.F., Wang S.X., Jiang L., et al. Pathologic predictors of renal outcome and therapeutic efficacy in IgA nephropathy: validation of the oxford classification. Clin J Am Soc Nephrol. 2011;6:2175–2184. doi: 10.2215/CJN.11521210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y., Sun L., Zhou S., et al. Intrarenal arterial lesions are associated with higher blood pressure, reduced renal function and poorer renal outcomes in patients with IgA nephropathy. Kidney Blood Press Res. 2018;43:639–650. doi: 10.1159/000489290. [DOI] [PubMed] [Google Scholar]
- 8.Herzenberg A.M., Fogo A.B., Reich H.N., et al. Validation of the Oxford classification of IgA nephropathy. Kidney Int. 2011;80:310–317. doi: 10.1038/ki.2011.126. [DOI] [PubMed] [Google Scholar]
- 9.Cai Q., Shi S., Wang S., et al. Microangiopathic lesions in IgA nephropathy: a cohort study. Am J Kidney Dis. 2019;74:629–639. doi: 10.1053/j.ajkd.2019.03.416. [DOI] [PubMed] [Google Scholar]
- 10.Timmermans S.A.M.E.G., Abdul-Hamid M.A., Potjewijd J., et al. C5b9 formation on endothelial cells reflects complement defects among patients with renal thrombotic microangiopathy and severe hypertension. J Am Soc Nephrol. 2018;29:2234–2243. doi: 10.1681/ASN.2018020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El Karoui K., Hill G.S., Karras A., et al. A clinicopathologic study of thrombotic microangiopathy in IgA nephropathy. J Am Soc Nephrol. 2012;23:137–148. doi: 10.1681/ASN.2010111130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faria B., Canão P., Cai Q., et al. Arteriolar C4d in IgA nephropathy: a cohort study. Am J Kidney Dis. 2020;76:669–678. doi: 10.1053/j.ajkd.2020.03.017. [DOI] [PubMed] [Google Scholar]
- 13.Chua J.S., Zandbergen M., Wolterbeek R., et al. Complement-mediated microangiopathy in IgA nephropathy and IgA vasculitis with nephritis. Mod Pathol. 2019;32:1147–1157. doi: 10.1038/s41379-019-0259-z. [DOI] [PubMed] [Google Scholar]
- 14.Leake R., Barnes D., Pinder S., et al. Immunohistochemical detection of steroid receptors in breast cancer: a working protocol. UK Receptor Group, UK NEQAS, The Scottish Breast Cancer Pathology Group, and The Receptor and Biomarker Study Group of the EORTC. J Clin Pathol. 2000;53:634–635. doi: 10.1136/jcp.53.8.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen P., Yu G., Zhang X., et al. Plasma galactose-deficient IgA1 and C3 and CKD progression in IgA nephropathy. Clin J Am Soc Nephrol. 2019;14:1458–1465. doi: 10.2215/CJN.13711118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Espinosa M., Ortega R., Sánchez M., et al. Association of C4d deposition with clinical outcomes in IgA nephropathy. Clin J Am Soc Nephrol. 2014;9:897–904. doi: 10.2215/CJN.09710913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chua J.S., Baelde H.J., Zandbergen M., et al. Complement factor C4d is a common denominator in thrombotic microangiopathy. J Am Soc Nephrol. 2015;26:2239–2247. doi: 10.1681/ASN.2014050429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bossi F., Tripodo C., Rizzi L., et al. C1q as a unique player in angiogenesis with therapeutic implication in wound healing. Proc Natl Acad Sci U S A. 2014;111:4209–4214. doi: 10.1073/pnas.1311968111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei M., Guo W.Y., Xu B.Y., et al. Collectin11 and complement activation in IgA nephropathy. Clin J Am Soc Nephrol. 2021;16:1840–1850. doi: 10.2215/CJN.04300321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhai Y.L., Meng S.J., Zhu L., et al. Rare variants in the complement factor H-related protein 5 gene contribute to genetic susceptibility to IgA nephropathy. J Am Soc Nephrol. 2016;27:2894–2905. doi: 10.1681/ASN.2015010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bu F., Maga T., Meyer N.C., et al. Comprehensive genetic analysis of complement and coagulation genes in atypical hemolytic uremic syndrome. J Am Soc Nephrol. 2014;25:55–64. doi: 10.1681/ASN.2013050453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaggl M., Aigner C., Csuka D., et al. Maternal and fetal outcomes of pregnancies in women with atypical hemolytic uremic syndrome. J Am Soc Nephrol. 2018;29:1020–1029. doi: 10.1681/ASN.2016090995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lafayette R.A., Rovin B.H., Reich H.N., Tumlin J.A., Floege J., Barratt J. Safety, tolerability and efficacy of Narsoplimab, a novel MASP-2 inhibitor for the treatment of IgA nephropathy. Kidney Int Rep. 2020;5:2032–2041. doi: 10.1016/j.ekir.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.