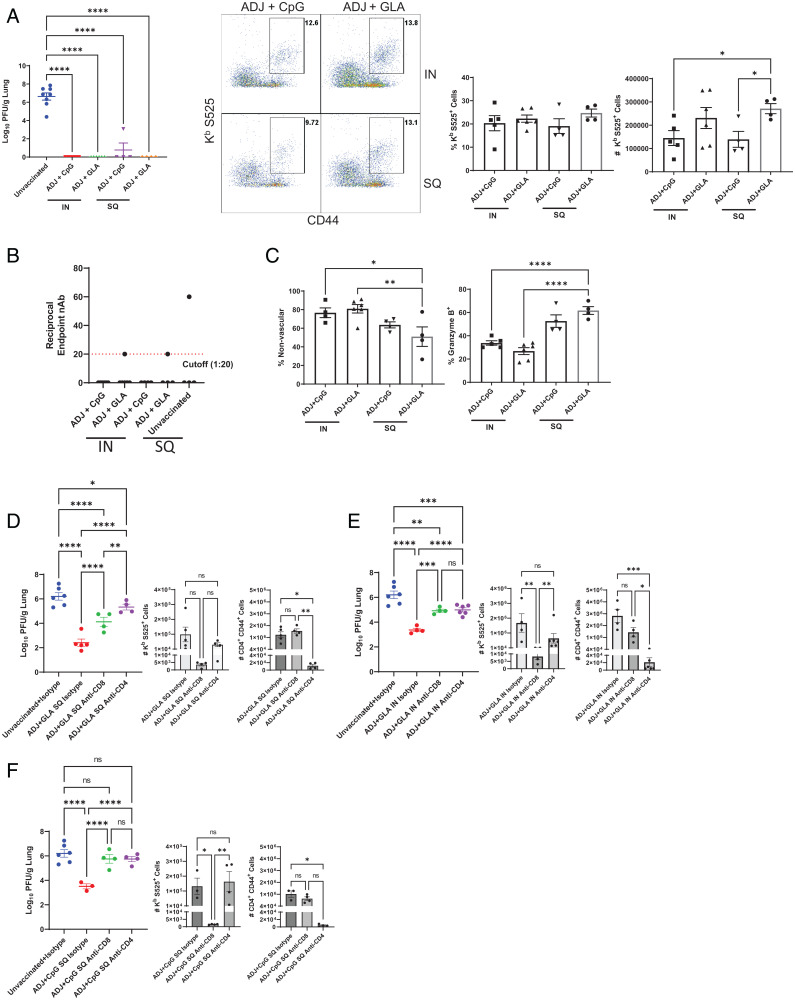
Fig. 6.
Vaccine-induced protective immunity to the SA variant of SARS-CoV-2 (B.1.351). Cohorts of 6- to 8-wk-old K18-ACE2tg mice were vaccinated twice with S protein of the WA strain of SARS-CoV-2, as described in Fig. 2. At 65 d after booster vaccination, when mice were ∼18 wk of age, they were challenged with the B.1.351 variant of SARS-CoV-2 virus; unvaccinated mice were challenged as controls. (A) Viral titers and S525-specific CD8 T cells were quantified in the lungs on day 5 after challenge. Graphs show percentages and total numbers of S525-specific CD8 T cells in lungs. (B) Neutralizing antibody titers to the SA B.1.351 variant in serum samples collected from mice at day 5 after challenge upon necropsy. (C) Percentages of vascular nonvascular (CD45.2−) and granzyme B+ cells among S525-specific CD8 T cells. (D–F) Cohorts of 6-wk-old K18-hACE2tg mice were vaccinated once with USA-WA1/2020 S protein formulated in ADJ+GLA SQ (D) or IN (E) route, or with ADJ+CpG SQ (F). Twenty-one days after vaccination, when mice were ∼9 wk of age, mice were treated IN and intravenously with anti-CD4 or anti-CD8 antibodies before and during challenge with the B.1.351 variant of SARS-CoV-2. Graphs show viral titers, S525-specific CD8+ and activated (CD44+) CD4 T cells in lungs on day 5 after challenge quantified by staining and flow cytometry, as described above. Asterisks indicate significance at *P < 0.05, **P < 0.005, ***P < 0.0005, and ****P < 0.00005; ns, not significant . Data in each graph indicate mean ± SEM.