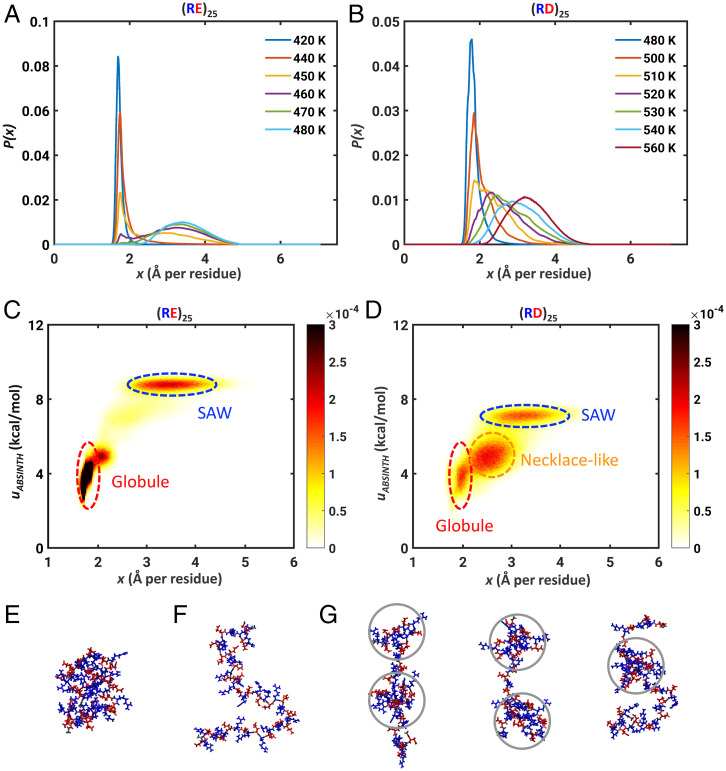
Fig. 4.
Asp residues modulate the two-state behaviors of polyampholytic IDPs. Calculated distributions P(x) for (A) (RE)25 and (B) (RD)25. Two-dimensional histograms p(x, uABSINTH) for (C) (RE)25 and (D) (RD)25. Here, uABSINTH is relative potential energy per residue, referenced to the system-specific lowest energy conformation. The histograms were computed as an average over unbiased replica exchange simulations at three temperatures near the transition temperature, namely, 450 K, 460 K, and 470 K for (RE)25 and 520 K, 530 K, and 540 K for (RD)25. The bin widths for x and uABSINTH are 0.004 Å and 0.02 kcal/mol per residue, respectively. (E) Snapshot of a representative globular conformation for (RE)25. (F) Snapshot of a representative self-avoiding-walk conformation for (RE)25. (G) Three snapshots, showing representative necklace-like conformations, with gray circles delineating the “pearls” formed by (RD)25. Lys is shown in blue and Glu/Asp residues are in red. Lys is shown in blue and Glu/Asp residues are in red.