Significance
Synapses are controlled by transsynaptic adhesion complexes that mediate bidirectional signaling between pre- and postsynaptic compartments. Long-term potentiation (LTP) of synaptic transmission is thought to enable synaptic modifications during memory formation, but the signaling mechanisms involved remain poorly understood. We show that binding of cerebellin-4 (Cbln4), a secreted ligand of presynaptic neurexin adhesion molecules, to neogenin-1, a postsynaptic surface protein known as a developmental netrin receptor, is essential for normal LTP at entorhinal cortex→dentate gyrus synapses in mice. Cbln4 and neogenin-1 are dispensable for basal synaptic transmission and not involved in establishing synaptic connections as such. Our data identify a netrin receptor as a postsynaptic organizer of synaptic plasticity that collaborates specifically with the presynaptic neurexin–ligand Cbln4.
Keywords: long-term potentiation, neurexin, neogenin 1, synaptic plasticity, hippocampus
Abstract
Five decades ago, long-term potentiation (LTP) of synaptic transmission was discovered at entorhinal cortex→dentate gyrus (EC→DG) synapses, but the molecular determinants of EC→DG LTP remain largely unknown. Here, we show that the presynaptic neurexin–ligand cerebellin-4 (Cbln4) is highly expressed in the entorhinal cortex and essential for LTP at EC→DG synapses, but dispensable for basal synaptic transmission at these synapses. Cbln4, when bound to cell-surface neurexins, forms transcellular complexes by interacting with postsynaptic DCC (deleted in colorectal cancer) or neogenin-1. DCC and neogenin-1 act as netrin and repulsive guidance molecule-a (RGMa) receptors that mediate axon guidance in the developing brain, but their binding to Cbln4 raised the possibility that they might additionally function in the mature brain as postsynaptic receptors for presynaptic neurexin/Cbln4 complexes, and that as such receptors, DCC or neogenin-1 might mediate EC→DG LTP that depends on Cbln4. Indeed, we observed that neogenin-1, but not DCC, is abundantly expressed in dentate gyrus granule cells, and that postsynaptic neogenin-1 deletions in dentate granule cells blocked EC→DG LTP, but again did not affect basal synaptic transmission similar to the presynaptic Cbln4 deletions. Thus, binding of presynaptic Cbln4 to postsynaptic neogenin-1 renders EC→DG synapses competent for LTP, but is not required for establishing these synapses or for otherwise enabling their function.
The Hebbian postulate (1) that long-term synaptic changes occur in ensembles of neurons that wire and fire together, and that, such changes form the basis for learning and memory, is the most widely accepted hypothesis for a cellular correlate of learning and memory. This hypothesis was potently supported by the finding that intense stimulation of neurons induces the long-term potentiation (LTP) of the strength of some synapses. LTP was originally discovered at entorhinal cortex→dentate gyrus (EC→DG) synapses (2), but studied most intensely at hippocampal Schaffer-collateral CA3 region→CA1 region (CA3→CA1) synapses. LTP manifests as an increase in synaptic strength induced by high-frequency stimulation in acute brain slices or in a behaving animal and is observed in multiple species and brain regions (3–7).
Work over nearly 50 years elucidated the molecular machinery that mediates the induction, expression, and maintenance of LTP (8–12). Most of this work focused on CA3→CA1 synapses, at which activation of N-methyl-D-aspartate receptors (NMDARs) during LTP induction causes Ca2+ influx that induces the postsynaptic recruitment of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs), thereby increasing synaptic strength. Despite vast progress in understanding CA3→CA1 LTP, major questions remain. For example, whether AMPARs are recruited by lateral diffusion after exocytosis or direct exocytosis at postsynaptic sites remains unclear, as does the question of why both Neuroligin-1 and LRRTMs, which are postsynaptic receptors for presynaptic neurexins, are required for LTP induction at CA3→CA1 synapses (13). Even less is known about LTP at synapses other than CA3→CA1 connections, in particular, at EC→DG synapses at which LTP was discovered. Here, LTP may have a different molecular basis, but this possibility has not been addressed.
DCC and neogenin-1 (Neo1) were identified as receptors for netrins and RGMa, which are essential axon-guidance molecules (14–17). Deletion of DCC causes defects in axonal projections in the developing brain (18). DCC or Neo1 deletions lead to behavioral symptoms associated with developmental disorders (19, 20), neuropsychiatric diseases (19, 21–29) with underlying deficits in axonal targeting (24, 28, 30, 31), and synaptic plasticity (23, 32, 33). Both DCC and Neo1 play additional roles in development (34) (reviewed in refs. 35–39). In view of the crucial role of DCC and Neo1 as netrin and RGMa receptors during development, it was a major surprise when Neo1 and DCC were also found to bind to cerebellin-4 (Cbln4), a secreted C1q-domain protein (40–42). Cbln4 belongs to a family of four synaptic proteins (Cbln1 to 4) that bind to presynaptic neurexins (43, 44). Of the four cerebellins, Cbln1, 2, and 4 are broadly expressed throughout the brain, whereas Cbln3 is only present in cerebellum and not secreted in the absence of Cbln1 or Cbln2 (44). Cbln1 and Cbln2, bound to presynaptic neurexins, interact with postsynaptic GluD1 and GluD2, which are surface receptors that are homologous to AMPARs and NMDARs and that transduce Cbln1/2-neurexin signals into a postsynaptic response (45–50). Cbln4, however, does not bind to GluDs (50). The interaction of Cbln4 with DCC and Neo1 suggested a possible role for DCC and Neo1 as transducers for a presynaptic neurexin–Cbln4 signal, similar to the role of GluDs in transducing presynaptic neurexin–Cbln1/2 signals, but the function of transsynaptic Cbln4–Dcc/Neo1 complexes is unknown.
Here, we show at EC→DG synapses that the binding of Cbln4 to Neo1 executes a critical role in synaptic plasticity. We demonstrate that Cbln4 and Neo1 are essential for induction of EC→DG LTP without being required for basal synaptic transmission. Cbln4 is only expressed in selected subsets of neurons in the brain; for example, it is present in the EC but absent from CA3 and CA1 region pyramidal neurons that exhibit classical NMDAR-dependent LTP. As a result, the Cbln4- and Neo1-dependent LTP we describe differs from CA3→CA1 LTP, which requires different sets of neurexin-based transsynaptic complexes, namely neurexin–neuroligin-1 and neurexin–LRRTM complexes. Thus, we elucidate a synaptic function for Cbln4–Neo1 complexes, suggesting a greater functional diversity of transsynaptic adhesion complexes in synaptic plasticity than previously envisioned.
Results
Cbln4 Is Highly Expressed in EC Neurons and Required for EC→DG LTP.
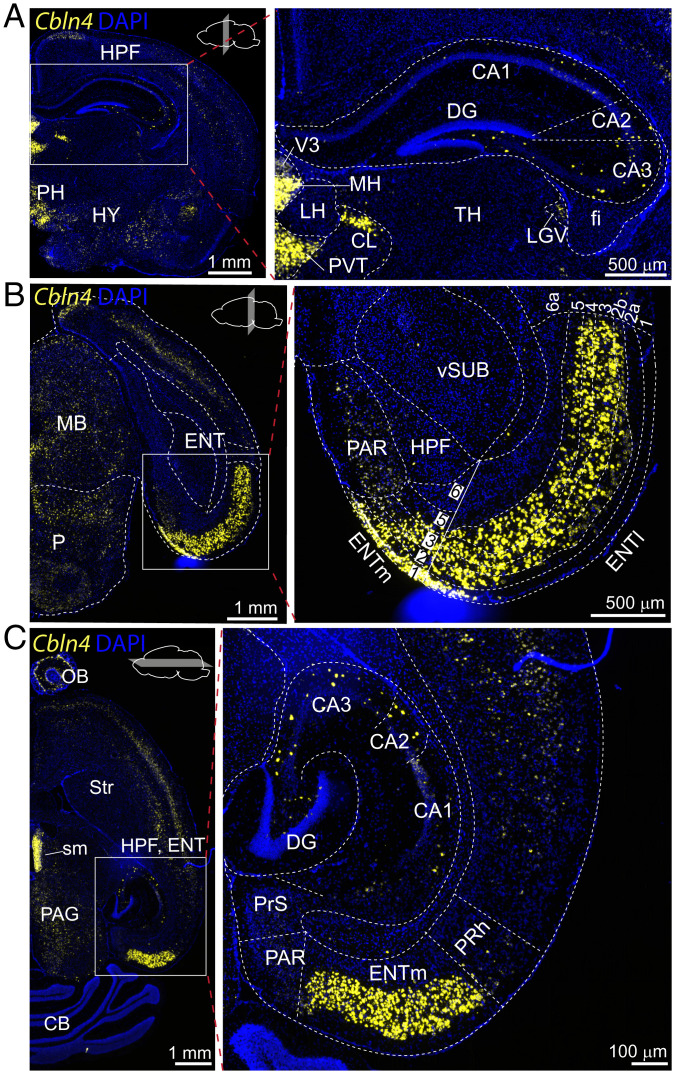
In an attempt to identify unique molecular features of the EC→DG circuit, we analyzed the expression of synaptic molecules in single-cell RNA-sequencing (RNA-seq) datasets from the murine cortex and hippocampus (https://portal.brain-map.org/atlases-and-data/rnaseq). Screening for the expression of 46 genes encoding transsynaptic signaling molecules in glutamatergic cell populations revealed a significant region-specific enrichment in layers L2/L3 of the EC of a member of the cerebellin family, Cbln4, but not of Cbln1, Cbln2, or Cbln3 (SI Appendix, Fig. S1). RNA fluorescent in-situ hybridization (RNA-FISH) uncovered high levels of Cbln4 expression in layers L1, 2, 3, and 5 but not in layer 6 of the medial EC (ENTm), and in layers L1, 2a, 2b (with gradient), 3, 4, and 5 but not in layer 6a of the lateral EC (ENTl) (Fig. 1 A–C). Principal cells in the hippocampus (dentate gyrus granule cells, CA1, 2, and 3 pyramidal cells) were devoid of Cbln4 expression, although hippocampal interneurons also expressed abundant levels of Cbln4 (Fig. 1 A and C). Outside of the hippocampal formation, Cbln4 was also expressed highly in the medial habenula as described previously (51).
Fig. 1.
Cbln4 expression is enriched in the EC. (A–C) Cbln4 mRNA expression revealed by single-molecule RNA-FISH in adult mouse. RNA-FISH assay on coronal hippocampus (A) and entorhinal cortical sections (B) and on horizontal brain sections of the entorhinal and hippocampal area (C). Boxed regions on the Left are enlarged at Right. (Abbreviations: HPF, hippocampal formation; fi, fimbria; LGv, ventral part of the lateral geniculate complex; CA, cornu ammonis; DG, dentate gyrus; PAR, parasubiculum; MB, midbrain; Vsub, ventral subiculum; V3, third ventricle; MH, medial habenula; LH, lateral habenula; PVT, paraventricular nucleus of the thalamus; PH, posterior hypothalamic nucleus; TH, thalamus; HY, hypothalamus; sm, stria medullaris; PAG, periaqueductal gray; Str, striatum, CB, cerebellum; OB, olfactory bulb).
Cerebellins are secreted adaptor molecules that connect presynaptic neurexins to postsynaptic receptors, thereby forming transsynaptic complexes (41, 46, 47, 49, 52–56). Only neurexins containing an insert in splice site 4 (SS4) bind to cerebellins. GluD1 and GluD2 (glutamate delta receptors, also called Gluδ1 and Gluδ2) function as postsynaptic receptors for Cbln1 and Cbln2 complexed to neurexins, but not for Cbln4 (note that Cbln3 is not expressed on its own) (50). In contrast, Neo1 and DCC act as receptors for Cbln4 (41), although no functional significance of this interaction was identified. The striking anatomical specificity of Cbln4 expression in the EC prompted us to hypothesize that Cbln4 could be involved in shaping EC→DG circuits via its interaction with neurexins that are also abundantly expressed in the EC (SI Appendix, Fig. S1).
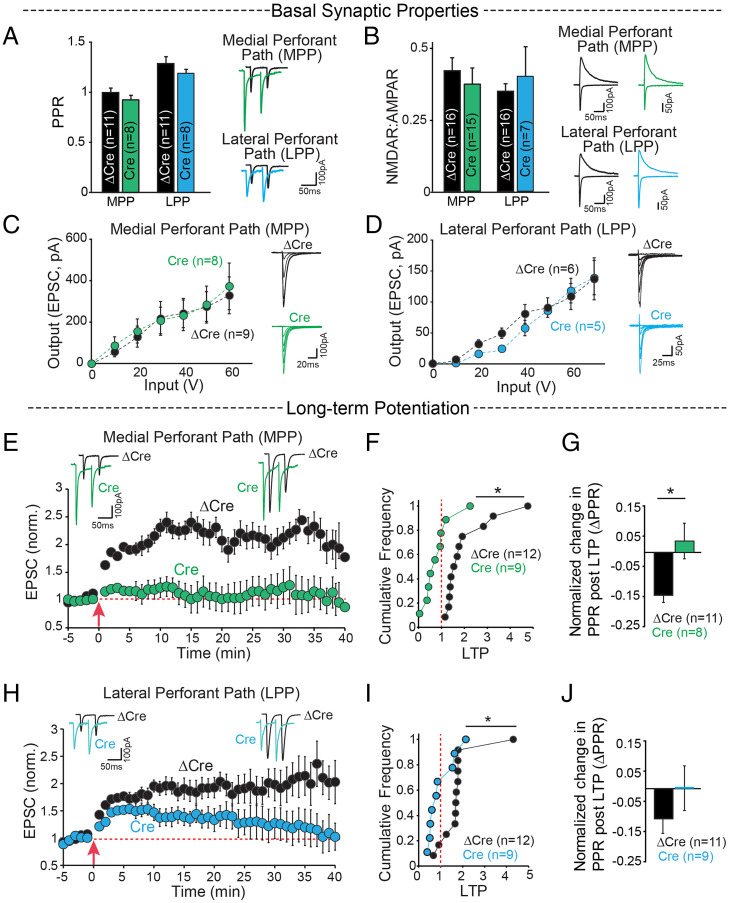
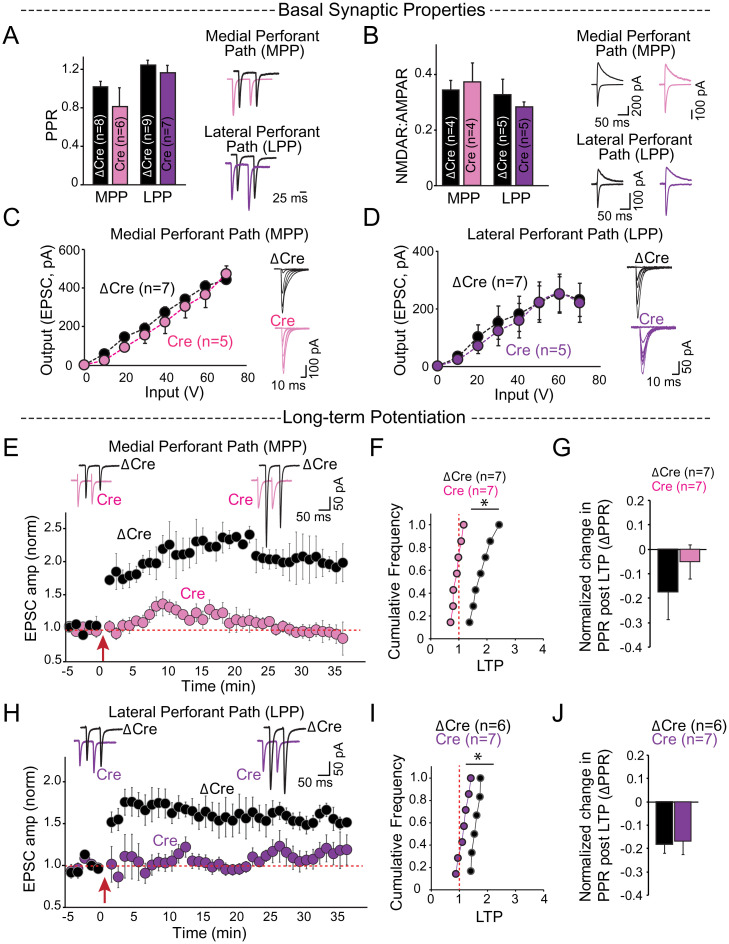
We thus set out to investigate whether Cbln4 is necessary for the function of the EC→DG circuit (SI Appendix, Fig. S2A). We stereotactically injected adeno-associated viruses (AAVs) expressing Cre-GFP (conditional knockout) or ΔCre-GFP (control) into the medial and lateral entorhinal cortices of Cbln4 conditional knockout (cKO) mice at postnatal day 18 to 21 (44, 57) (SI Appendix, Fig. S2B). Two weeks later, we cut hippocampal slices and performed whole-cell voltage-clamp recordings from DG granule cells and measured evoked synaptic responses elicited by stimulation of EC inputs in order to test the role of Cbln4 in EC→DG synapses. In these experiments, we used axonal stimulation of medial and lateral EC efferents to activate the medial perforant path (MPP) and lateral perforant path (LPP), respectively. EC→DG synapses formed by the MPP and LPP have different presynaptic properties that produce distinct paired-pulse ratios (PPRs) (58). The presynaptic deletion of Cbln4 had no effect on any basal synaptic activity of MPP or LPP synapses, including their PPR (Fig. 2A), NMDAR to AMPAR ratios (Fig. 2B), or input–output relationships of AMPAR excitatory postsynaptic currents (EPSCs) (Fig. 2 C and D). These results indicate that the presynaptic Cbln4 deletion does not change the number or basal properties of the synapses of MPP and LPP EC→DG circuits.
Fig. 2.
Cbln4 deletion in the EC selectively blocks LTP at EC→DG synapses. (A–J) Whole-cell voltage-clamp recordings from dentate gyrus granule cells for stimulation of inputs from the medial entorhinal cortex through the MPP and lateral entorhinal cortex through the LPP from control (ΔCre) and entorhinal cortex Cbln4 KO (Cre) mouse brain slices. (A) Summary graphs (Left) and sample traces (Right) of PPRs show no significant difference between Cre-and ΔCre-injected mice in MPP (ΔCre 0.99 ± 0.05, Cre 0.93 ± 0.05) and LPP (ΔCre 1.28 ± 0.07, Cre 1.19 ± 0.04). (B) NMDA-receptor to AMPA-receptor ratios. MPP: ΔCre 0.42 ± 0.05, Cre 0.37 ± 0.06; LPP: ΔCre 0.35 ± 0.03, Cre 0.40 ± 0.12. (C and D) Summary graphs with sample traces of input–output relationships of AMPAR EPSCs for incremental stimulation intensities show no significant difference between the two conditions. (E–J) Outcome of LTP in EC Cre- and ΔCre-injected Cbln4 cKO mice. (E) Sample EPSC traces before and after LTP induction (Top) and time course (Bottom) for LTP induction in the MPP of control (ΔCre) and entorhinal cortex Cbln4 KO (Cre) mouse brain slices. (F) Cumulative distribution of normalized LTP, ΔCre 2.03 ± 0.31, Cre 0.83 ± 0.21, *P = 0.007. (G) Normalized change in PPR at 35 to 40 min following LTP, ΔCre −0.144 ± 0.02, Cre 0.04 ± 0.06, *P = 0.02). (H–J) Same as E–G, except for LTP induction in the LPP. (H) Sample EPSC traces before and after LTP induction (Top) and time course (Bottom) for LTP induction in the LPP of control (ΔCre) and entorhinal cortex Cbln4 KO (Cre) mouse brain slice. (I) ΔCre 2.11 ± 0.25, Cre 1.11 ± 0.24, *P = 0.04. (J) ΔCre −0.1 ± 0.04, Cre 0.003 ± 0.076.
Next, we asked whether Cbln4 might contribute to the plasticity, instead of the basic operation of EC→DG synapses, and tested the role of Cbln4 in EC→DG LTP (2). In ΔCre-injected animals, high-frequency stimulation of EC afferents produced a sustained, approximately twofold potentiation of EPSCs (30 to 40 min post-LTP induction) in both the MPP and the LPP pathways. In Cre-injected mice, no significant potentiation was observed in MPP (Fig. 2 E–G) or LPP EC→DG synapses (Fig. 2 H–J). Moreover, LTP induced a decrease in the PPR of MPP synapses in control mice, but not in mice with a deletion of Cbln4 in the EC (Fig. 2G), suggesting a presynaptic contribution to LTP (59), whereas the PPR in LPP synapses was unchanged (Fig. 2J). Overall, these results indicate that expression of Cbln4 in the EC is not required for the connectivity of EC→DG synapses, but is essential for their LTP.
Cbln4 Connects Presynaptic Neurexins with Postsynaptic Neo1 into a Transsynaptic Complex.
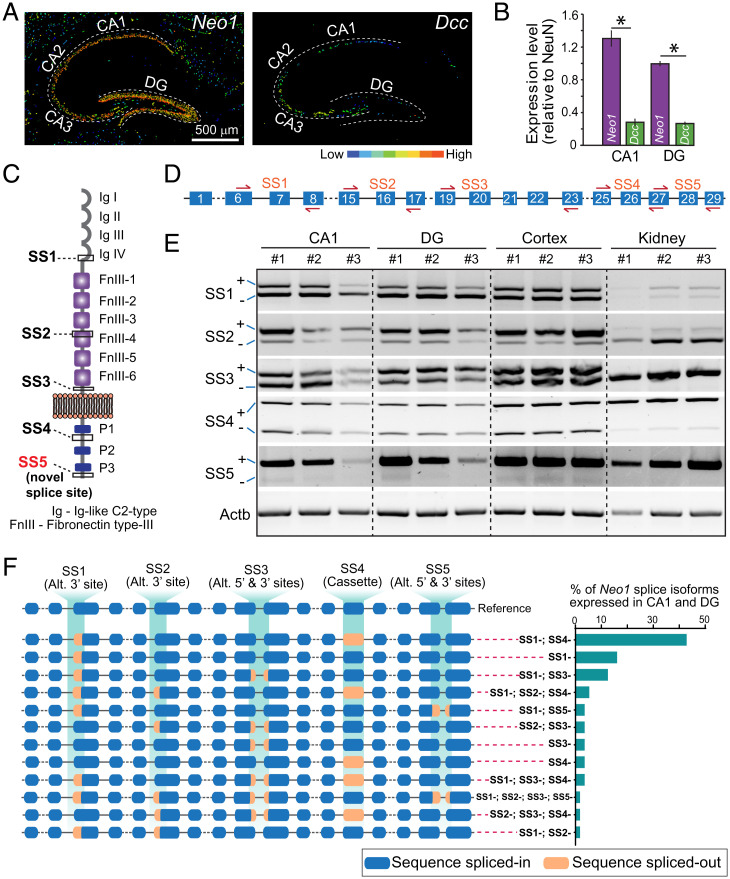
To elucidate whether Neo1 or DCC might act as a postsynaptic receptor for presynaptic Cbln4, we analyzed RNA in-situ hybridization data from the Allen Brain Atlas and performed qRT-PCR validations. These assays showed that Neo1 and DCC are both expressed in the DG and the CA1 region, but that in these brain regions Neo1 mRNA levels are approximately three- to four-fold higher than DCC mRNA levels (Fig. 3 A and B). Further analyses of single-cell RNA-seq datasets revealed that Neo1 is highly enriched in both of the two major dentate gyrus granule cell populations (GRC1 and GRC2), establishing it as a plausible receptor candidate for Cbln4 (SI Appendix, Fig. S3). GRC1 and GRC2 neurons are very similar, but differ in gene expression profiles, with Bhlhe22, Tmem114, and Ntf3 serving as markers for GRC1 and C1ql2 and Nr3c2 as markers for GRC2 (60).
Fig. 3.
Neo1 but not DCC is highly expressed in the hippocampus and has multiple splice isoforms arising from many splicing events. (A) Expression view of Allen Brain Atlas RNA in-situ hybridization data for Neo1 and DCC mRNA levels in the hippocampal region shows high level of Neo1 expression in the DG. (B) Relative mRNA levels in the DG and CA1 of Neo1 (0.99 ± 0.02; 1.30 ± 0.09) and DCC (0.27 ± 0.02, 0.28 ± 0.04), *P < 0.05. (C) Protein domain architecture with known (SS1–SS4) and novel splice (SS5) sites in Neo1. (D) Exon (numbered boxes) and intron (lines) organization of Neo1 gene with primers (red arrows) to amplify each splice site (SS1–SS5). (E) Agarose gel with RT-PCR products amplified using splice junction primers show pattern of Neo1 splice form expression in the CA1, DG, and whole cortex. Kidney mRNA used as a control (in three replicates). (F) Exon–intron structure of Neo1 splice variants cloned from CA1 and DG cDNA shows distinct splicing events with % of expression of each splice variant revealed by full-length DNA sequencing. Alt., alternative 5′ and 3′ splice sites; cassette, cassette exon.
Since Neo1 is alternatively spliced (61), we asked which Neo1 variants are expressed in the hippocampus. To address this question, we cloned full-length Neo1 from hippocampal cDNA. We identified an unexpectedly rich diversity of alternatively spliced Neo1 transcripts, including a previously unknown site of alternative splicing (SS5) in the intracellular sequence (Fig. 3 C–E). In the hippocampus, Neo1 mRNAs lacking SS1 and SS4 (Neo1-SS1− and Neo1-SS4−) were the most abundant transcripts (∼40%). Splicing events such as alternative 3′, 5′ splice acceptor sites and combined alternative 3′ and 5′ sites events further contribute to the heterogeneity of Neo1 splice isoforms (Fig. 3F).
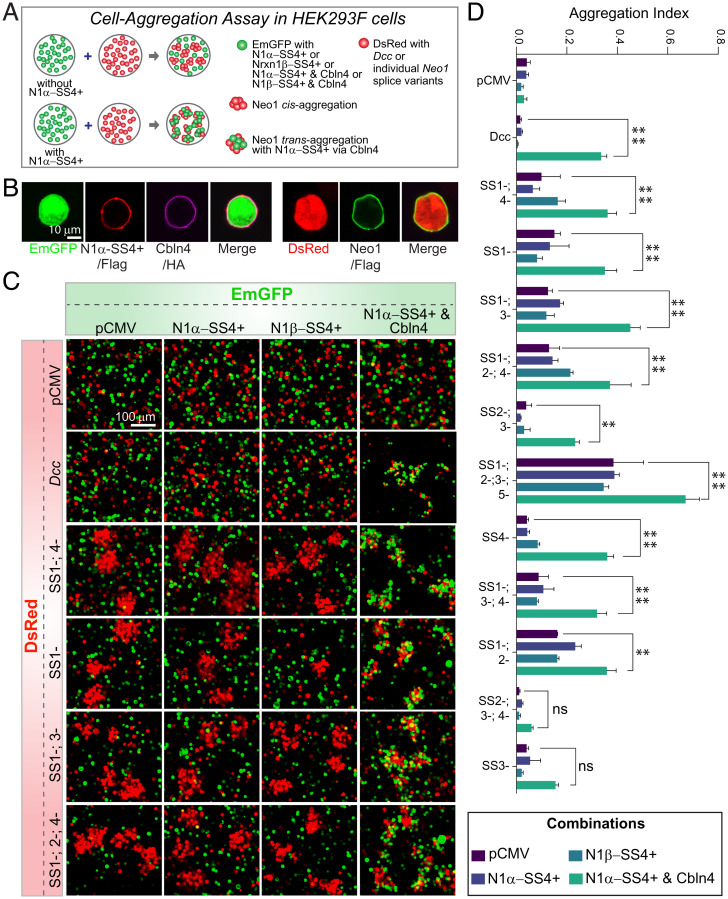
To confirm the binding of Neo1 and DCC to Cbln4 and to test whether binding is controlled by alternative splicing similar to the binding of neurexins to Cbln4, we coexpressed Cbln4 with Nrxn1α-SS4+ in freestyle HEK293F cells (Fig. 4 A and B) and mixed these cells with HEK293F cells expressing DCC or various splice variants of Neo1 (Fig. 4C and SI Appendix, Fig. S4). In the absence of cells expressing Cbln4 and Nrxn1α-SS4+, cells expressing various Neo1 splice variants formed clumps, consistent with a homophilic, likely nonspecific interaction (Fig. 4C). When cells expressing Nrxn1α-SS4+ or Nrxn1β-SS4+ alone were added, they did not attach to the DCC- or Neo1-expressing cells. However, when cells coexpressing Nrxn1α-SS4+ with Cbln4 were added, they avidly formed heterophilic aggregates with the Neo1-expressing cells (Fig. 4C). Quantifications showed that DCC and all Neo1 splice variants except for Neo1-SS2−, 3−, 4−, and SS3− variants were active in these assays (Fig. 4D). Thus, Neo1 binds in trans to Cbln4/Nrxn1α-SS4+ complexes, forming bona fide adhesion complexes.
Fig. 4.
Neo1 binds to Cbln4/Neurexin-1 complexes. (A) Schematic of cell aggregation assays in HEK293T cells to validate interaction of Neo1 and DCC with Cbln4. (B) Immunocytochemical validation of EmGFP (Emerald GFP), DsRed expression, and surface labeling of N1α−SS4+ and representative Neo1–SS1−;SS4− (using anti-Flag antibody) and Cbln4 (using anti-HA antibody) in HEK293F cells. (C) Confocal images of cell aggregation assay showing a strong aggregation of green cells (coexpressing EmGFP, N1α-SS4+ and Cbln4) with red cell clusters (coexpressing DsRed and various Neo1 splice variants or Dcc). (D) Quantification of the aggregation index from the data in C and SI Appendix, Fig. S4. The binding of DCC and various Neo1 splice variants to Nrxn1α–SS4+/Cbln4 complexes was assessed, using pCMV (empty vector), Nrxn1β–SS4+ alone, and Nrxn1α–SS4+ alone as controls. Data are means ± SEM from two to three replicate experiments. Statistical significance was calculated by Tukey’s multiple comparisons test (two-way ANOVA). ****P < 0.0001, **P < 0.01, ns P > 0.05.
Neo1 Expression in the DG Is Necessary for EC→DG LTP.
The abundant expression of Neo1 in the DG and its robust interaction with Cbln4 suggest that Neo1 is the postsynaptic receptor for presynaptic Cbln4/Nrx1α-SS4+ complexes, raising the possibility that Neo1 mediates the essential Cbln4 signaling in the induction of EC→DG LTP. To test this hypothesis, we selectively deleted Neo1 in the DG by stereotactically injecting AAVs expressing Cre or ΔCre (as a control) into the DG of Neo1 cKO mice (SI Appendix, Fig. S5). Again, the basal properties of EC→DG synapses, including PPR in the MPP and LPP synapses on granule cells (Fig. 5A), NMDAR-to-AMPAR ratios (Fig. 5B), and input–output relationships of AMPAR EPSCs (Fig. 5 C and D) were unchanged following deletion of Neo1 in the DG. However, postsynaptic deletion of Neo1 in the DG severely impaired LTP in EC→DG synapses of both MPP (Fig. 5 E–G) and LPP connections (Fig. 5 H–J), similar to the presynaptic deletion of Cbln4 in the EC (Fig. 1). Thus, the postsynaptic deletion of Neo1 produces the same impairment in long-term synaptic plasticity as the presynaptic deletion of Cbln4, suggesting that the binding of presynaptic Cbln4 to postsynaptic Neo1 controls the competence of synapses to be modified by LTP without affecting the functional assembly of these synapses.
Fig. 5.
Postsynaptic deletion of Neo1 in the DG selectively ablates LTP at EC→DG synapses. Whole-cell voltage-clamp recordings from dentate gyrus granule cells for stimulation of inputs from the medial entorhinal cortex through the MPP and lateral entorhinal cortex through the LPP from control (ΔCre) and dentate gyrus Neo1 cKO (Cre) mouse brain slices. (A) Summary graph (Left) and sample traces (Right) of PPRs show no significant difference between the two conditions. MPP: ΔCre 1.02 ± 0.07, Cre 0.87 ± 0.08; LPP: ΔCre 1.26 ± 0.04, Cre 1.21 ± 0.04. (B) Summary graph (Left) and sample traces (Right) of NMDAR–AMPAR ratios show no significant difference between the two conditions. MPP: ΔCre 0.34 ± 0.03, Cre 0.37 ± 0.07; LPP: ΔCre 0.32 ± 0.06, Cre 0.28 ± 0.02. (C and D) Summary graph (Left) and sample traces (Right) of input–output relationships of AMPAR EPSCs for incremental stimulation intensities show no significant difference between the two conditions. (E–J) LTP is blocked in both MPP and LPP following postsynaptic deletion of Neo1 in the dentate gyrus. (E) Sample EPSC traces before and after LTP induction (Top) and time course of LTP (Bottom) for stimulation of the MPP in control (ΔCre) and Neo1 cKO (Cre) mouse brain slices. (F) Cumulative distribution of normalized LTP, ΔCre 1.82 ± 0.14, n = 7. Cre 0.92 ± 0.06, n = 7, *P = 0.0001 and (G) normalized change in PPR, ΔCre = −0.17 ± 0.11, Cre −0.05 ± 0.07, 35 to 40 min following LTP induction. (H and I) Same as in E and F except for stimulation of the LPP. (H) Sample EPSC traces before and after LTP induction (Top) and time course of LTP (Bottom) for stimulation of the LPP in control (ΔCre) and Neo1 cKO (Cre) mouse brain slices. (I) Cumulative distribution of normalized LTP, ΔCre 1.58 ± 0.06, Cre 1.15 ± 0.7, *P = 0.001 and (J) normalized change in PPR, ΔCre −0.1 ± 0.04, Cre 0.003 ± 0.076. Data are presented as mean ± SEM, *P < 0.05 (two-tailed t test).
Discussion
Although Neo1 and DCC are known to play essential roles in development by serving as netrin receptors, the functional significance of their binding of Cbln4 remained unclear (40, 42). Moreover, despite large numbers of studies on Cbln1 and Cbln2, the function of Cbln4 has been scarcely examined. Here, we address these two gaps in our knowledge by demonstrating that presynaptic Cbln4 and postsynaptic Neo1 are both essential for the induction of LTP at EC→DG synapses without being required for synaptic connectivity or basal synaptic transmission (Fig. 6). These results thus suggest that Cbln4 binding to Neo1 activates a signaling pathway in DG granule cells that is selectively essential for LTP, thereby assigning a function to the Cbln4/Neo1 complex. Strikingly, the Cbln4/Neo1 complex was required for LTP in both the lateral and the medial perforant path of EC→DG synapses. This is unexpected because LTP in these two pathways was considered mechanistically different since LTP is associated with a change in paired-pulse ratio in the MPP but not the LPP pathway (59), but their common dependence on the Cbln4/Neo1 complex suggests that these two EC efferent synapses are mechanistically more similar than envisioned.
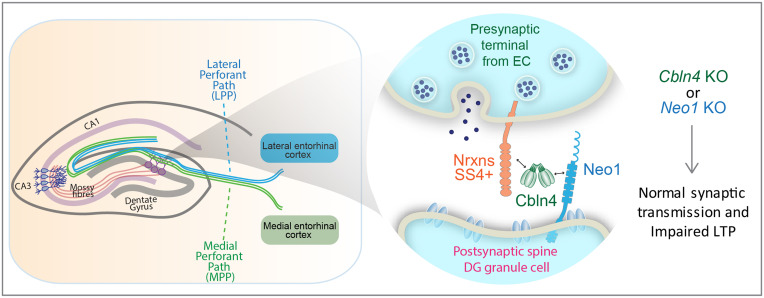
Fig. 6.
Schematic of the role of Cbln4 and Neo1 as transsynaptic adhesion molecules that control long-term synaptic plasticity at EC→DG synapses. Cbln4, a dimer of tetramers binds to presynaptic neurexins containing an insert in SS4+ (neurexins) via its N-terminal stalk domain and to postsynaptic Neo1 via its C-terminal C1q domain, thereby dimerizing Neo1 as a potential mechanism of activation (not shown). The Neurexin/Cbln4/Neo1 complex is essential not for building and maintaining synapses or for synaptic transmission as such, but for rendering these synapses competent for LTP, thereby providing a mechanistic feature to dentate gyrus LTP, the type of LTP at which this form of long-term synaptic plasticity was discovered.
The requirement of Cbln4 and Neo1 for EC→DG LTP reveals a critical role for transsynaptic signaling mediated by neurexin-based complexes in LTP at EC→DG synapses that parallels the requirement for neurexin-based neuroligin and LRRTM complexes for traditional LTP at CA3→CA1 synapses (13, 62–64). Both the postsynaptic neurexin ligands neuroligin-1 (13, 62) and LRRTM1/2 (63, 64) were found to be required for CA3→CA1 LTP. Moreover, we observed that presynaptic neurexin-3 is essential for LTP in yet another synapse, the CA1→subiculum synapse (65). In these synapses, Cbln4 is not detectably present, and Cbln1 and Cbln2 were shown by deletions to also not be essential for LTP (45), suggesting that distinct neurexin-based transsynaptic signaling mechanisms may render different synapses competent for LTP.
As extensively documented for Cbln1 and Cbln2, cerebellins perform a variety of functions depending on the synaptic architecture in which they are embedded. In the cerebellum, deletion of Cbln1 leads to a partial reduction in parallel-fiber synapses on Purkinje cells that is secondary to a change in synaptic transmission, which includes a loss of long-term depression at these synapses (66, 67). The deletion of GluD2, the postsynaptic receptor for Cbln1 at cerebellar parallel-fiber synapses, caused identical phenotypes (68). In contrast, in the parafascicular nucleus of the thalamus, deletion of Cbln1 produced an increase in synapse numbers in thalamus→striatum connections (69). Moreover, deletion of the Cbln1 receptor GluD1 in the striatum puzzlingly decreased synapse numbers and increased the quantal size of AMPAR responses at thalamus→striatum synapses (70). Furthermore, deletion of Cbln2 in the medial habenular nucleus that projects to the interpeduncular nucleus caused an impairment in synaptic transmission without initially affecting synapse numbers, although the number of excitatory synapses was decreased later on (51). In contrast, deletion of Cbln2 from the dorsal raphe nucleus had no effect on synapse numbers but produced hyperactive, aggressive, and compulsive behaviors in mice that resemble symptoms underlying Tourette’s syndrome and schizophrenia (71). In the hippocampal subiculum, finally, Cbln2 was found to regulate the postsynaptic AMPAR and NMDAR content via binding to postsynaptic GluD1, again without affecting synapse numbers (45). The overall picture that emerges from these studies indicates that Cbln1 and Cbln2 primarily regulate synapse properties via binding to GluD1 and GluD2, and that in some but not all synapses, such as cerebellar parallel-fiber synapses, this leads to a secondary loss of synaptic connections.
In contrast to Cbln1 and Cbln2, the function of Cbln4 has not been studied extensively. In mice, deletion of Cbln4 along with Cbln1/2 induced seizures and abnormal motor behaviors but had little effect on synapse numbers (57). Deletion of Cbln4 in the medial habenula→interpeduncular nucleus circuit increased anxiety, but again had no effect on synapse numbers (51). Experiments using shRNA-mediated knockdowns suggested that a decrease in Cbln4 expression in the somatosensory cortex (72) and the hippocampus (73) reduces the number of inhibitory synapses. Puzzlingly, however, in the somatosensory cortex, Cbln4 was shown to act by interacting with postsynaptic GluD1 (72) even though biophysical studies show that Cbln4 does not bind to GluD1 (50, 74), but instead interacts with DCC and Neo1 as discussed above (40, 42).
Although our results establish a role for the Cbln4/Neo1 complex in EC→DG LTP, our study has clear limitations. First, we did not identify the presynaptic neurexin isoforms that operate in LTP at EC→DG synapses. Second, while we established a role for Neo1 as the postsynaptic interacting partner for Cbln4 in mediating EC→DG LTP, the signaling mechanisms downstream of Neo1 that mediate LTP remain unclear (36). Third, we did not test the functional importance of the extensive alternative splicing of Neo1 that we observed, which may add a further regulatory component to EC→DG synapses, although it doesn’t appear to control Cbln4 binding. Despite these unanswered questions, however, the identification of a general role of different neurexin-based transsynaptic complexes in long-term synaptic plasticity and the definition of the synaptic function for Cbln4 and Neo1 in mature brain represent a basis on which future studies on signaling mechanisms in plasticity can be advanced.
Methods
Mice.
All mouse experiments were performed in accordance with protocols approved by the Administrative Panel on Laboratory Animal Care at Stanford University.
Viruses and In Vivo Injections.
AAVs produced as described (75) were injected into the EC of Cbln4 and Neo1 cKO mice at P18 to P21 as described (75); see also detailed methods in SI Appendix, SI Methods).
Slice Electrophysiology.
Slice electrophysiology was performed at P35 to P40 using published methods (76). Visually identified DG granule cells were whole-cell voltage-clamped and stimulated using theta glass pipettes filled with artificial cerebrospinal fluid (ACSF) that were placed in the MPP or LPP. All excitatory synaptic recordings were made in the presence of picrotoxin (0.1 mM). Cells were held at −60 mV to record AMPAR EPSCs while stimulating afferent inputs at 0.1 Hz. NMDAR currents were calculated by measuring the amplitude of the dual component EPSC at +40 mV at 40 to 50 ms following the peak. The NMDAR/AMPAR ratio of the EPSCs was calculated as the ratio of NMDAR EPSC at +40 mV and AMPAR EPSC at −60 mV. Paired pulse ratios were calculated as a ratio of the peak amplitudes of the EPSC-2 and EPSC-1 evoked delivering two stimulations at an interval of 50 ms. To measure the input–output curves of MPP and LPP stimulation, 20 to 30 episodes of AMPAR EPSCs to incremental stimulus strength were measured for each pathway. LTP was induced by two trains of high-frequency stimulation (100 Hz, 1 s) separated by 10 s, while cells were depolarized to 0 mV.
Analyses of mRNA Expression Patterns.
RNA-FISH was performed as described (77). qRT-PCR was performed with microdissected CA1 and DG tissues or whole cortex or kidneys essentially as described (77).
Cell Aggregation Assays.
Cell aggregation assays were performed as described (75) but modified to a six-well format (see detailed methods in SI Appendix, SI Methods.
Statistics.
All data are presented as means ± SEMs. Statistical significance was calculated between the two genotypes using two-tailed t tests (*P < 0.05).
Supplementary Material
Acknowledgments
We thank Erica Seigneur for sharing Cbln4 mice. The work for this study that was performed at Stanford University was supported by grants from the National Institute of Mental Health (MH052804 to T.C.S.), the European Molecular Biology Organization (ALTF 803-2017 to K.L.-A.), and the Larry L. Hillblom Foundation (2020-A-016-FEL to K.L.-A.).
Footnotes
Reviewers: T.B., Yale School of Medicine; P.C., Albert Einstein College of Medicine; and S.S., Universitatsklinikum Bonn Klinik fur Epileptologie.
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2123421119/-/DCSupplemental.
Data Availability
All study data are included in the article and/or supporting information. See data file in the supporting information.
References
- 1.Hebb D. O., The Organization of Behavior: A Neuropsychological Theory(Wiley, New York, NY, 1949). [Google Scholar]
- 2.Bliss T. V., Lomo T., Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J. Physiol. 232, 331–356 (1973). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang S., et al. , Associative Hebbian synaptic plasticity in primate visual cortex. J. Neurosci. 34, 7575–7579 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kupfermann I., Castellucci V., Pinsker H., Kandel E., Neuronal correlates of habituation and dishabituation of the gill-withdrawal reflex in Aplysia. Science 167, 1743–1745 (1970). [DOI] [PubMed] [Google Scholar]
- 5.Löwel S., Singer W., Selection of intrinsic horizontal connections in the visual cortex by correlated neuronal activity. Science 255, 209–212 (1992). [DOI] [PubMed] [Google Scholar]
- 6.Margrie T. W., Rostas J. A., Sah P., Long-term potentiation of synaptic transmission in the avian hippocampus. J. Neurosci. 18, 1207–1216 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teyler T. J., et al. , Long-term potentiation of human visual evoked responses. Eur. J. Neurosci. 21, 2045–2050 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basu J., Siegelbaum S. A., The corticohippocampal circuit, synaptic plasticity, and memory. Cold Spring Harb. Perspect. Biol. 7, a021733 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diering G. H., Huganir R. L., The AMPA receptor code of synaptic plasticity. Neuron 100, 314–329 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malenka R. C., Bear M. F., LTP and LTD: An embarrassment of riches. Neuron 44, 5–21 (2004). [DOI] [PubMed] [Google Scholar]
- 11.Volianskis A., et al. , Long-term potentiation and the role of N-methyl-D-aspartate receptors. Brain Res. 1621, 5–16 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Díaz-Alonso J., Nicoll R. A., AMPA receptor trafficking and LTP: Carboxy-termini, amino-termini and TARPs. Neuropharmacology 197, 108710 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang M., et al. , Conditional ablation of neuroligin-1 in CA1 pyramidal neurons blocks LTP by a cell-autonomous NMDA receptor-independent mechanism. Mol. Psychiatry 22, 375–383 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keino-Masu K., et al. , Deleted in Colorectal Cancer (DCC) encodes a netrin receptor. Cell 87, 175–185 (1996). [DOI] [PubMed] [Google Scholar]
- 15.Matsunaga E., Chédotal A., Repulsive guidance molecule/neogenin: A novel ligand-receptor system playing multiple roles in neural development. Dev. Growth Differ. 46, 481–486 (2004). [DOI] [PubMed] [Google Scholar]
- 16.Vielmetter J., et al. , Molecular characterization of human neogenin, a DCC-related protein, and the mapping of its gene (NEO1) to chromosomal position 15q22.3-q23. Genomics 41, 414–421 (1997). [DOI] [PubMed] [Google Scholar]
- 17.Vielmetter J., Kayyem J. F., Roman J. M., Dreyer W. J., Neogenin, an avian cell surface protein expressed during terminal neuronal differentiation, is closely related to the human tumor suppressor molecule deleted in colorectal cancer. J. Cell Biol. 127, 2009–2020 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fazeli A., et al. , Phenotype of mice lacking functional Deleted in colorectal cancer (Dcc) gene. Nature 386, 796–804 (1997). [DOI] [PubMed] [Google Scholar]
- 19.Jamuar S. S., et al. , Biallelic mutations in human DCC cause developmental split-brain syndrome. Nat. Genet. 49, 606–612 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jain R. A., Bell H., Lim A., Chien C. B., Granato M., Mirror movement-like defects in startle behavior of zebrafish dcc mutants are caused by aberrant midline guidance of identified descending hindbrain neurons. J. Neurosci. 34, 2898–2909 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cuesta S., et al. , DCC-related developmental effects of abused- versus therapeutic-like amphetamine doses in adolescence. Addict. Biol. 25, e12791 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cuesta S., et al. , Non-contingent exposure to amphetamine in adolescence recruits miR-218 to regulate Dcc expression in the VTA. Neuropsychopharmacology 43, 900–911 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glasgow S. D., et al. , Pre- and post-synaptic roles for DCC in memory consolidation in the adult mouse hippocampus. Mol. Brain 13, 56 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manitt C., et al. , dcc orchestrates the development of the prefrontal cortex during adolescence and is altered in psychiatric patients. Transl. Psychiatry 3, e338 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reynolds L. M., et al. , Amphetamine in adolescence disrupts the development of medial prefrontal cortex dopamine connectivity in a DCC-dependent manner. Neuropsychopharmacology 40, 1101–1112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torres-Berrío A., et al. , DCC confers susceptibility to depression-like behaviors in humans and mice and is regulated by miR-218. Biol. Psychiatry 81, 306–315 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vosberg D. E., Leyton M., Flores C., The Netrin-1/DCC guidance system: Dopamine pathway maturation and psychiatric disorders emerging in adolescence. Mol. Psychiatry 25, 297–307 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vosberg D. E., et al. , Mesocorticolimbic connectivity and volumetric alterations in DCC mutation carriers. J. Neurosci. 38, 4655–4665 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun D., et al. , Neogenin, a regulator of adult hippocampal neurogenesis, prevents depressive-like behavior. Cell Death Dis. 9, 8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chua J. Y., Ng S. J., Yagensky O., Wanker E. E., Chua J. J. E., FEZ1 forms complexes with CRMP1 and DCC to regulate axon and dendrite development. eNeuro 8, ENEURO.0193-20.2021 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reynolds L. M., et al. , DCC receptors drive prefrontal cortex maturation by determining dopamine axon targeting in adolescence. Biol. Psychiatry 83, 181–192 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horn K. E., et al. , DCC expression by neurons regulates synaptic plasticity in the adult brain. Cell Rep. 3, 173–185 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Sun X. D., et al. , Neogenin in amygdala for neuronal activity and information processing. J. Neurosci. 38, 9600–9613 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robinson R. A., et al. , Simultaneous binding of Guidance Cues NET1 and RGM blocks extracellular NEO1 signaling. Cell 184, 2103–2120.e31 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Vries M., Cooper H. M., Emerging roles for neogenin and its ligands in CNS development. J. Neurochem. 106, 1483–1492 (2008). [DOI] [PubMed] [Google Scholar]
- 36.Siebold C., Yamashita T., Monnier P. P., Mueller B. K., Pasterkamp R. J., RGMs: Structural insights, molecular regulation, and downstream signaling. Trends Cell Biol. 27, 365–378 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilson N. H., Key B., Neogenin: One receptor, many functions. Int. J. Biochem. Cell Biol. 39, 874–878 (2007). [DOI] [PubMed] [Google Scholar]
- 38.Cole S. J., Bradford D., Cooper H. M., Neogenin: A multi-functional receptor regulating diverse developmental processes. Int. J. Biochem. Cell Biol. 39, 1569–1575 (2007). [DOI] [PubMed] [Google Scholar]
- 39.Anderson R. B., Cooper H. M., Jackson S. C., Seaman C., Key B., DCC plays a role in navigation of forebrain axons across the ventral midbrain commissure in embryonic xenopus. Dev. Biol. 217, 244–253 (2000). [DOI] [PubMed] [Google Scholar]
- 40.Haddick P. C., et al. , Defining the ligand specificity of the deleted in colorectal cancer (DCC) receptor. PLoS One 9, e84823 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joo J. Y., et al. , Differential interactions of cerebellin precursor protein (Cbln) subtypes and neurexin variants for synapse formation of cortical neurons. Biochem. Biophys. Res. Commun. 406, 627–632 (2011). [DOI] [PubMed] [Google Scholar]
- 42.Wei P., et al. , The Cbln family of proteins interact with multiple signaling pathways. J. Neurochem. 121, 717–729 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miura E., Iijima T., Yuzaki M., Watanabe M., Distinct expression of Cbln family mRNAs in developing and adult mouse brains. Eur. J. Neurosci. 24, 750–760 (2006). [DOI] [PubMed] [Google Scholar]
- 44.Seigneur E., Südhof T. C., Cerebellins are differentially expressed in selective subsets of neurons throughout the brain. J. Comp. Neurol. 525, 3286–3311 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dai J., Patzke C., Liakath-Ali K., Seigneur E., Südhof T. C., GluD1 is a signal transduction device disguised as an ionotropic receptor. Nature 595, 261–265 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hirai H., et al. , Cbln1 is essential for synaptic integrity and plasticity in the cerebellum. Nat. Neurosci. 8, 1534–1541 (2005). [DOI] [PubMed] [Google Scholar]
- 47.Ibata K., et al. , Activity-dependent secretion of synaptic organizer Cbln1 from lysosomes in granule cell axons. Neuron 102, 1184–1198.e10 (2019). [DOI] [PubMed] [Google Scholar]
- 48.Matsuda K., et al. , Cbln1 is a ligand for an orphan glutamate receptor delta2, a bidirectional synapse organizer. Science 328, 363–368 (2010). [DOI] [PubMed] [Google Scholar]
- 49.Uemura T., et al. , Trans-synaptic interaction of GluRdelta2 and Neurexin through Cbln1 mediates synapse formation in the cerebellum. Cell 141, 1068–1079 (2010). [DOI] [PubMed] [Google Scholar]
- 50.Zhong C., et al. , Cbln1 and Cbln4 are structurally similar but differ in GluD2 binding interactions. Cell Rep. 20, 2328–2340 (2017). [DOI] [PubMed] [Google Scholar]
- 51.Seigneur E., Polepalli J. S., Südhof T. C., Cbln2 and Cbln4 are expressed in distinct medial habenula-interpeduncular projections and contribute to different behavioral outputs. Proc. Natl. Acad. Sci. U.S.A. 115, E10235–E10244 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elegheert J., et al. , Structural basis for integration of GluD receptors within synaptic organizer complexes. Science 353, 295–299 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ito-Ishida A., et al. , Cbln1 regulates rapid formation and maintenance of excitatory synapses in mature cerebellar Purkinje cells in vitro and in vivo. J. Neurosci. 28, 5920–5930 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ito-Ishida A., et al. , Presynaptically released Cbln1 induces dynamic axonal structural changes by interacting with GluD2 during cerebellar synapse formation. Neuron 76, 549–564 (2012). [DOI] [PubMed] [Google Scholar]
- 55.Mishina M., Uemura T., Yasumura M., Yoshida T., Molecular mechanism of parallel fiber-Purkinje cell synapse formation. Front. Neural Circuits 6, 90 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tao W., Díaz-Alonso J., Sheng N., Nicoll R. A., Postsynaptic δ1 glutamate receptor assembles and maintains hippocampal synapses via Cbln2 and neurexin. Proc. Natl. Acad. Sci. U.S.A. 115, E5373–E5381 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seigneur E., Südhof T. C., Genetic ablation of all cerebellins reveals synapse organizer functions in multiple regions throughout the brain. J. Neurosci. 38, 4774–4790 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Colino A., Malenka R. C., Mechanisms underlying induction of long-term potentiation in rat medial and lateral perforant paths in vitro. J. Neurophysiol. 69, 1150–1159 (1993). [DOI] [PubMed] [Google Scholar]
- 59.Min M. Y., Asztely F., Kokaia M., Kullmann D. M., Long-term potentiation and dual-component quantal signaling in the dentate gyrus. Proc. Natl. Acad. Sci. U.S.A. 95, 4702–4707 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.La Manno G., et al. , Molecular architecture of the developing mouse brain. Nature 596, 92–96 (2021). [DOI] [PubMed] [Google Scholar]
- 61.Keeling S. L., Gad J. M., Cooper H. M., Mouse Neogenin, a DCC-like molecule, has four splice variants and is expressed widely in the adult mouse and during embryogenesis. Oncogene 15, 691–700 (1997). [DOI] [PubMed] [Google Scholar]
- 62.Wu X., et al. , Neuroligin-1 signaling controls LTP and NMDA receptors by distinct molecular pathways. Neuron 102, 621–635.e3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bhouri M., et al. , Deletion of LRRTM1 and LRRTM2 in adult mice impairs basal AMPA receptor transmission and LTP in hippocampal CA1 pyramidal neurons. Proc. Natl. Acad. Sci. U.S.A. 115, E5382–E5389 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Soler-Llavina G. J., et al. , Leucine-rich repeat transmembrane proteins are essential for maintenance of long-term potentiation. Neuron 79, 439–446 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aoto J., Martinelli D. C., Malenka R. C., Tabuchi K., Südhof T. C., Presynaptic neurexin-3 alternative splicing trans-synaptically controls postsynaptic AMPA receptor trafficking. Cell 154, 75–88 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yuzaki M., Two classes of secreted synaptic organizers in the central nervous system. Annu. Rev. Physiol. 80, 243–262 (2018). [DOI] [PubMed] [Google Scholar]
- 67.Südhof T. C., Synaptic neurexin complexes: A molecular code for the logic of neural circuits. Cell 171, 745–769 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yuzaki M., Aricescu A. R., A GluD coming-of-age story. Trends Neurosci. 40, 138–150 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kusnoor S. V., Parris J., Muly E. C., Morgan J. I., Deutch A. Y., Extracerebellar role for Cerebellin1: Modulation of dendritic spine density and synapses in striatal medium spiny neurons. J. Comp. Neurol. 518, 2525–2537 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu J., et al. , Striatal glutamate delta-1 receptor regulates behavioral flexibility and thalamostriatal connectivity. Neurobiol. Dis. 137, 104746 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Seigneur E., Wang J., Dai J., Polepalli J., Südhof T. C., Cerebellin-2 regulates a serotonergic dorsal raphe circuit that controls compulsive behaviors. Mol. Psychiatry 26, 7509–7521 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fossati M., et al. , Trans-synaptic signaling through the glutamate receptor delta-1 mediates inhibitory synapse formation in cortical pyramidal neurons. Neuron 104, 1081–1094.e7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chacón P. J., et al. , Cerebellin 4, a synaptic protein, enhances inhibitory activity and resistance of neurons to amyloid-β toxicity. Neurobiol. Aging 36, 1057–1071 (2015). [DOI] [PubMed] [Google Scholar]
- 74.Yasumura M., et al. , Glutamate receptor δ1 induces preferentially inhibitory presynaptic differentiation of cortical neurons by interacting with neurexins through cerebellin precursor protein subtypes. J. Neurochem. 121, 705–716 (2012). [DOI] [PubMed] [Google Scholar]
- 75.Sando R., Jiang X., Südhof T. C., Latrophilin GPCRs direct synapse specificity by coincident binding of FLRTs and teneurins. Science 363, eaav7969 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Polepalli J. S., et al. , Modulation of excitation on parvalbumin interneurons by neuroligin-3 regulates the hippocampal network. Nat. Neurosci. 20, 219–229 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liakath-Ali K., Südhof T. C., The perils of navigating activity-dependent alternative splicing of neurexins. Front. Mol. Neurosci. 14, 659681 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or supporting information. See data file in the supporting information.