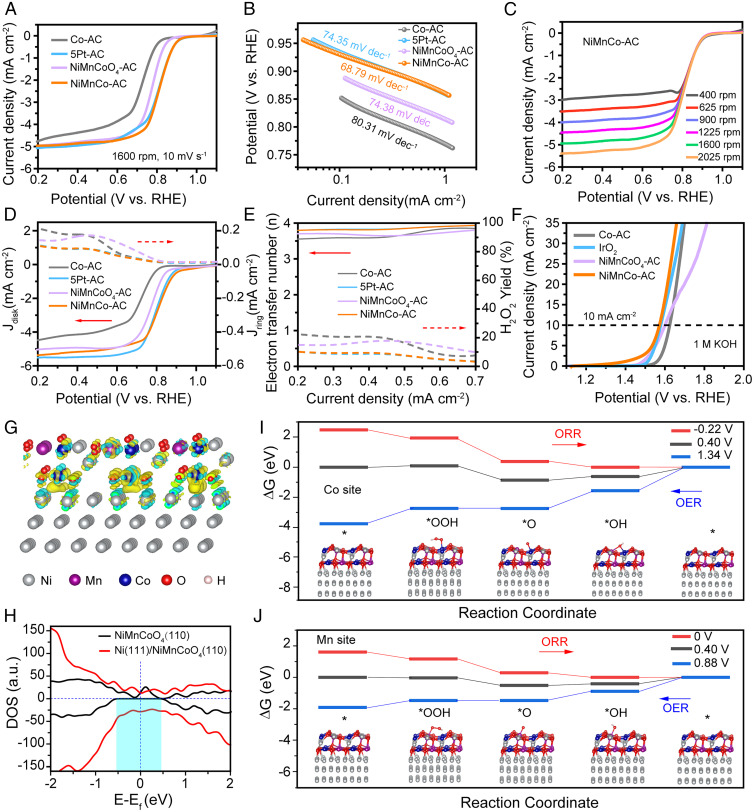
Fig. 4.
The electrocatalytic performance of the NiMnCo-AC catalyst. (A) ORR polarization curves of the Co-AC, NiMnCo-AC, NiMnCoO4-AC, and 5Pt-AC catalysts. (B) Tafel plots derived from the ORR curves. (C) ORR curves of the NiMnCo-AC catalyst in 0.1 M KOH with increasing rotating rates from 400 to 2,025 rpm. (D) RRDE curves of the Co-AC, NiMnCo-AC, NiMnCoO4-AC, and 5Pt-AC catalysts. (E) Percentage of peroxide production and the electron transfer number based on the RRDE results. (F) OER polarization curves of the Co-AC, NiMnCo-AC, NiMnCoO4-AC, and IrO2 catalysts in 1 M KOH at the rotating rate of 1,600 rpm. (G) Interfacial electron transfer in Ni(111)@NiMnCoO4(110). Yellow and cyan iso-surface represents electron accumulation and electron depletion; the iso-surface value is 0.01 e Å−3. (H) The DOS on NiMnCoO4(110) and Ni(111)@NiMnCoO4(110). (I and J), Gibbs free energy diagrams of ORR/OER on the Co sites (I) and Mn sites (J) of Ni(111)@NiMnCoO4(110).