Table 4.
Compounds having confirmed inhibition of TMPRSS2 in both biochemical assays and in the cellular assay.
| Biochemical (fluorogenic) | Biochemical (fluorogenic counterassay) | Biochemical (mass spectrometry) | SARS-CoV-2 PP (Delta) | SARS-CoV-2 PP (WA1 + D614G) | Calu-3 (cytotoxicity) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound Name | Structure | AC50 (μM) | Max Response (%) | AC50 (μM) | AC50 (μM) | Max Response (%) | AC50 (μM) | Max Response (%) | AC50 (μM) | Max Response (%) | Status for Human Use |
| Nafamostat mesilate |
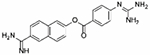
|
0.00011 | −1.9 | 0.0002 | 0.0014 | −100% | 0.0064 | −100% | N/D | 0% | Approved |
| Camostat |

|
0.0062 | −5 | 0.0055 | 0.059 | −92% | 0.05 | −95% | N/D | 0% | Approved |
| PCI-27433 |
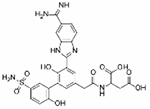
|
1.8 | 61.3 | 1.4 | 15.85 | −66% | 16.5 | −66% | N/D | 0% | Clinical Trials |
| Cmpd 92 (Meyer) |
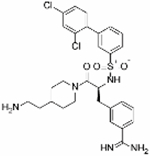
|
0.41 | −5.6 | 0.28 | 3.98 | 92% | 2.05 | −70% | N/D | 0% | N/T |
| Cmpd 114 (Meyer) |
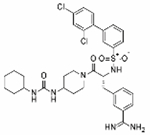
|
0.57 | −6.2 | 0.26 | 15.27 | 99% | 5.35 | −88% | N/D | 0% | N/T |
| Otamixaban |
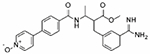
|
1.64 | −1 | 1.23 | 18.01 | 54% | 20 | −50% | N/D | 0% | Clinical Trials |
N/D = Not Detected
N/T = Not Tested
