Abstract
Abnormal fear and anxiety can manifest as psychiatric disorders. The bed nucleus of the stria terminalis (BNST) is implicated in sustained responding to, or anticipation of, an aversive event which can be expressed as anticipatory anxiety. The BLA is also active during anticipatory anxiety and sends projections to the BNST. However, little is known about the role for BLA neurons that project to BNST (BLA-BNST) in anticipatory anxiety in rodents. To address this, we tested whether chemogenetic inactivation of the BLA-BNST pathway attenuates sustained conditioned responses produced by anticipation of an aversive stimulus. For comparison, we also assessed BLA-BNST inactivation during social interaction, which is sensitive to unlearned anxiety. We found that BLA-BNST inactivation reduced conditioned sustained freezing and increased social behaviors, but surprisingly, only in males. To determine whether sex differences in BLA-BNST neuronal activity contribute to the differences in behavior, we used in vivo and ex vivo electrophysiological approaches. In males, BLA-BNST projection neurons were more active and excitable, which coincided with a smaller after-hyperpolarization current (IAHP) compared with other BLA neurons; whereas in females, BLA-BNST neurons were less excitable and had larger IAHP compared with other BLA neurons. These findings demonstrate that activity of BLA-BNST neurons mediates conditioned anticipatory anxiety-like behavior in males. The lack of a role of BLA-BNST in females in this behavior, possibly because of low excitability of these neurons, also highlights the need for caution when generalizing the role of specific neurocircuits in fear and anxiety.
SIGNIFICANCE STATEMENT Anxiety disorders disproportionately affect women. This hints toward sex differences within anxiety neurocircuitry, yet most of our understanding is derived from male rodents. Furthermore, debilitating anticipation of adverse events is among the most severe anxiety symptoms, but little is known about anticipatory anxiety neurocircuitry. Here we demonstrated that BLA-BNST activity is required for anticipatory anxiety to a prolonged aversive cue, but only in males. Moreover, BLA-BNST neurons are hypoactive and less excitable in females. These results uncover BLA-BNST as a key component of anticipatory anxiety circuitry, and cellular differences may explain the sex-dependent role of this circuit. Uncovering this disparity provides evidence that the assumed basic circuitry of an anxiety behavior might not readily transpose from males to females.
Keywords: anxiety, BLA, BNST, sex differences
Introduction
Survival is dependent on the ability to learn about cues in the environment that predict threat. However, anxiety disorders are characterized by exaggerated responding to these cues (Jovanovic and Ressler, 2010; Norrholm et al., 2015; Norrholm and Jovanovic, 2018). These pathologic manifestations of fear and anxiety underlie disorders that impact almost 30% of the population with a disproportionate predominance in women (Kessler et al., 2005; Bandelow and Michaelis, 2015; Remes et al., 2016). An improved understanding of the neurocircuitry underlying these disorders could uncover more targeted treatment approaches.
Heightened fear and anxiety, and decreases in social behavior that characterize PTSD (Brunello et al., 2001; Fani et al., 2012; Fenster et al., 2018; Goode et al., 2019), can be observed in rodents after stress or other aversive experiences (Perusini and Fanselow, 2015; Rajbhandari et al., 2018). Fear and anxiety can be distinguished based on temporal characteristics, with fear defined by rapid response to a transient discrete cue. In some cases, this may shift to a state of anxiety characterized as a sustained response during ambiguity or in anticipation of a threat. While a neurocircuit from the BLA to central amygdala has been firmly established in fear, less is understood about the neurocircuitry of anxiety. Hyperactivity of the bed nucleus of the stria terminalis (BNST) in humans is associated with PTSD, and activation of the BNST is noted during anticipatory anxiety when the environment is unpredictable (Herry et al., 2007; Daldrup et al., 2016; Brinkmann et al., 2017; Awasthi et al., 2020). The BNST plays a key role in contextual fear learning and the expression of sustained fear responses (Davis et al., 2010; Lange et al., 2017; Torrisi et al., 2018; Sasaki Russell et al., 2020), as well as during long duration cues that provoke persistent avoidance or freezing (Waddell et al., 2006; Goode et al., 2019). Measurement of sustained responding during a prolonged cue provides a way to measure persistent anticipatory anxiety that can be dissociated from context (Waddell et al., 2006).
The BLA is a major input to the BNST and is involved in contextual and sustained fear learnings (Zimmerman and Maren, 2011; Herrmann et al., 2016), is activated by unpredictable threat and is hyperactive in anxiety disorders (Kim et al., 2011; Michely et al., 2020). Prior studies present equivocal evidence for the BLA-BNST in unlearned behaviors that are sensitive to anxiety (Kim et al., 2013), but it is not known whether this pathway is required for sustained anticipatory anxiety produced by a prolonged cue. Further, the BNST is a sexually dimorphic brain region, and that may contribute to sex differences in the expression of fear behaviors. For example, female rodents exhibit less contextual fear conditioning and higher exploration in tasks that reflect anxiety compared with males (Zimmerberg and Farley, 1993; Palanza, 2001; Dalla and Shors, 2009), which may be attributed to sex differences in neuronal activity of the BNST (Salvatore et al., 2018). In addition, there are sex differences in BLA neuronal activity which associate with differences in fear behavior (Blume et al., 2017, 2019). However, most prior work examining the role of BLA and BNST in prolonged anxiety has been in males, and it is not known whether the activity and function of BLA-BNST are similar between sexes.
Here, we examined the contribution of the BLA-BNST pathway in anticipatory anxiety by assessing sustained conditioned responding to a long duration cue. A secondary goal of this work was to examine whether there are sex differences in anticipatory anxiety and to characterize the circuitry contributing to this behavior. This was accomplished by inhibiting BLA-BNST neurons during conditioned prolonged anxiety and unlearned behaviors sensitive to anxiety using intersectional chemogenetic methods. To test the neural correlates for sex differences in sustained anxiety, we used electrophysiological approaches to measure the activity and excitability of BLA-BNST neurons.
Materials and Methods
All procedures were approved by the Rosalind Franklin University Animal Care and Use Committee, and complied with the Guide for the care and use of laboratory animals (National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals, 2011). All efforts were made to reduce the total number of animals used, minimize animal suffering, and use alternative approaches to replace animals when available.
Animals and determination of estrous cycle stage
Adult (>60 d postnatal [PND]) male and female Sprague Dawley rats (Charles River Laboratories) were same-sex group housed (2 or 3/cage) in the Rosalind Franklin University Biological Research Facility where they were allowed access to food and water ad libitum. The animal housing room was on a 12:12 reverse light/dark cycle, and ambient temperature (68°F-79°F) and humidity (30%-70%) were maintained. All animals were acclimated to the facility for at least 7 d before any experimental procedures. Following acclimation to the facilities, estrous cyclicity was determined in female rats via daily vaginal lavages. Males were handled daily for a similar amount of time. Vaginal cytology was examined by light microscopy to determine daily estrous stage as previously described (Blume et al., 2017). After at least two consecutive 4 d estrous cycles, animals were used for experimental procedures. All experiments were performed with females in either proestrus (high estrogen) or metestrus (low estrogen). Any females not exhibiting a 4 d estrous cycle were excluded from experimental procedures (N = 5).
Stereotaxic injection surgery for chemogenetics
In order to inhibit BLA neurons that project to the BNST, a combinatorial genetic approach was used, which used an adeno-associated viral (AAV) vector with the plasmid containing the Cre-dependent DREADD-Gi (AAV5-hSyn-DIO-hM4D-mCherry; Bryan Roth, UNC Vector Core) and the retrogradely transported canine adenovirus Type 2 vector containing Cre-recombinase (CAV2-Cre; Plateforme de Vectorologie de Montpellier, Institut de Génétique Moléculaire de Montpellier) injected into the BLA and BNST, respectively. In brief, rats were anesthetized with isoflurane (5% induction, 1%-3% maintenance; Patterson Veterinary), secured into blunt ear bars of a stereotaxic device (David Kopf Instruments), and injected with meloxicam to provide analgesia (Meloxicam, 1 mg/kg, s.c., Patterson Veterinary). The top of the head was shaved, and the surgical area was cleaned with betadine (Patterson Veterinary) and 70% ethanol before 1% lidocaine was administered at the incision site (0.2 ml, Patterson Veterinary). An incision was made, and the skull surface was exposed. Burr holes were drilled above the BLA (±5.0 mm lateral, −3.0 mm caudal, and 8.3 mm ventral from bregma) and BNST (±1.5-1.6 mm lateral, 0.0 mm caudal, and 6.9 mm ventral from bregma). Bilateral injections (500 nl/site) were infused in the BNST (CAV2-Cre) and BLA (AAV5-hSyn-DIO-hM4D-mCherry) through a 34 gauge needle attached to a 10 µl Hamilton syringe driven by a stereotaxic automated injector (World Precision Instruments) at a rate of 100 nl/min. After the injections were completed, the injector remained in situ for 5 min before removal, and the wound was closed with surgical staples. Rats were monitored and regained full mobility before being returned to their home cage. Postoperative care was provided for 48 h following surgery during which rats received a daily analgesic (1 mg/kg meloxicam, s.c.; Patterson Veterinary) and were monitored daily for signs of distress. Rats recovered for at least 21 d to allow the retrograde transport of CAV2-Cre from the BNST and subsequent expression of DREADD-Gi-mCherry in the BLA. During this time, estrous cyclicity was monitored daily, and behavior experiments began after at least 2 consecutive estrous cycles were recorded and 21 d of recovery had passed.
Immunohistochemical validation of in vivo DREADD expression
Upon conclusion of experiments with virus-injected animals, rats were deeply anesthetized with isoflurane (5%) and transcardially perfused with vascular buffer (PBS) with procaine (Sigma) and heparin (Alfa Aesar) followed by 4% PFA. Animals were subsequently decapitated, the brain was removed and stored overnight in 4% PFA, then transferred to 0.1 m PBS and stored at 4°C until sectioned on a vibratome (Leica Microsystems). To characterize viral injection placements and to verify AAV-DREADD and CAV2-Cre expression, brain sections (40 μm) were immunostained for the mCherry tag of the AAV plasmid and Cre-recombinase, respectively. Free-floating brain sections were washed (5 times for 5 min) in 0.1 m PBS (pH 7.3-7.4) and then incubated in 1% H2O2 in 0.1 m PBS for 15 min at room temperature to quench endogenous peroxidase activity. Sections were then washed (3 times for 5 min) in 0.1 m PBS, incubated for 3 h at room temperature in immunocytochemistry buffer (0.1 m PBS containing 0.2% gelatin, 0.01% thimerosal, and 0.002% neomycin, pH 7.5) with 5% Normal Donkey Serum/0.3% Triton (blocking buffer) to reduce nonspecific binding. The tissue was then incubated in primary antibodies directed against Cre-recombinase (1:2K, rabbit anti-Cre-recombinase; catalog #908001, BioLegend) and mCherry (1:4K chicken anti-mCherry; catalog #CPCA-mCherry, EnCor, Biotechnology) in blocking buffer for 48-72 h at 4°C. Sections were then washed (10 times for 5 min) in immunocytochemistry buffer and incubated for 3 h at room temperature in blocking buffer with fluorescent-tagged secondary antibodies targeting rabbit (1:250 goat anti-rabbit-488, catalog #A11008, Invitrogen) and chicken (1:250 donkey anti chicken-594, catalog #703-585-155, Jackson ImmunoResearch Laboratories). Following incubation, the sections were washed (4 times for 5 min) in TBS and subsequently mounted onto gelatinized slides. Coverslips were applied using Fluromount (Sigma Aldrich). Sections were imaged (Nikon E600 microscope), and Cre-recombinase and mCherry expression was mapped onto rat brain atlas sections (Paxinos and Watson, 2007) to identify the location of neurons transduced with CAV2-Cre-recombinase and AAV5-hSyn-DIO-hM4D(Gi)-mCherry. Only animals in which bilateral expression of mCherry was confined to the BLA were included in data analysis. Images were acquired (Nikon E600 microscope, Nikon Instruments), postacquisition analysis was performed using Fluoview software (Olympus America), and images were formatted for figures using Adobe Photoshop (Adobe Systems).
Behavioral experiments
Open field (OF) test
The OF (black opaque, 100 cm × 100 cm) test was performed in dim white light (20-25 lux) and dim red light for 5 min. Rats were randomly divided into groups and given an injection of either clozapine-N-oxide (CNO; 1 mg/kg in 0.9% saline; i.p., Tocris Bioscience) or 0.9% saline (1 ml/kg; i.p.) before the task (45-55 min). The field was thoroughly cleaned with 70% ethanol between rats. Behavior in the OF was recorded using infrared-sensitive cameras (DBK 27AUR0135, The Imaging Source) connected to a computer (Dell E6500) and analyzed offline using ANY-Maze (version 4.99 z; Stoelting). The OF was divided into 16 boxes with the 4 center squares defined as the center area. The total distance traveled and central area exploration in the field were quantified and compared between groups. Latency to enter the center, time, entries, and distance traveled in the center were calculated to assess exploratory behavior, while total distance traveled was calculated as an index of overall locomotive behavior. Data points that were >2 SDs above or below the group mean were removed from analysis (N = 2 saline males).
Social interaction (SI) test
At least 1 d after the OF task, rats were then subjected to the SI test 2 times (96 h/one estrous cycle apart): once after vehicle (0.9% saline 1 ml/kg, i.p.) and once after CNO (1 mg/kg, i.p.) injections (45-55 min before SI) in a counterbalanced manner. This experimental design with repeated SI was chosen to allow accurate within-animal assessment of the effects of BLA-BNST inactivation and reduce potential variability in baseline social interaction. All SI trials occurred in the same OF apparatus and lighting conditions as above. A separate group of non–virus-treated rats was used to control for nonspecific effects of CNO. During SI, rats were placed in the OF arena with a novel rat that was the same sex and of comparable weight (<50 g difference), and allowed to interact with each other for 10 min. As above, behavior was recorded using an infrared-sensitive camera connected to a computer (Dell E6500) and analyzed offline. The field was thoroughly cleaned with 70% ethanol between sessions. Social interactions were defined by sniffing (body and anogenital), close following and physically pushing the head or snout against any part of the novel rat's body. The time spent interacting, number of interactions, and latency to interact were hand-scored from videos by an experimenter blinded to the conditions. Four days after this SI trial, individual rats were given the opposite treatment (vehicle or CNO) from the first trial and placed with another novel rat in the OF box. Comparisons between individual animal saline and CNO trials were made to determine change in social behavior following inhibition of BLA-BNST neurons.
Prolonged cued fear conditioning
Prolonged cued fear conditioning was performed at least 48 h after the last social interaction trial. For conditioning, all rats were placed in a chamber (23.5 inch L × 28 inch W × 12.5 inch H, MED-VFC, Med Associates) with stainless-steel grid flooring housed inside a sound-attenuating cabinet, and a camera mounted on the wall of the cabinet recorded behavior (VID-CAM-MONO-5, Med Associates). Chambers were cleaned with 70% ethanol between animals. On the first day, rats were allowed to explore the chambers freely (2 min). Following this 2 min habituation period, a prolonged tone (5 kHz, 80 dB, 8 min) was presented during which 5 footshocks (0.3 mA; 0.5 s; at varied intervals) were administered. This was followed by a 1 min recovery period without the tone or footshocks. Rats were returned to their home cage until testing 4 d later (to measure at the same stage of estrus). Testing occurred in the same chambers as training, but with a different context (altered wall pattern, colors, odors, and flooring). The testing phase consisted of a 2 min habituation period followed by the same 8 min tone presentation as the training, but without footshocks. Rats remained in the chamber for 1 min at the end of the tone and were then returned to the home cage. The percent time spent freezing during the habituation, tone presentation, and recovery period were used as an index of conditioned fear to a prolonged cue. For experiments in which DREADD-treated rats were included, either CNO (1 mg/kg, i.p.) or 0.9% saline (1 ml/kg; i.p.) was administered 45-55 min before the training or testing session. A subset of rats (N = 18) received no footshocks during training sessions to confirm freezing behavior observed was not because of prolonged tone presentation alone. Another subset of naive rats (N = 22) received either CNO (1 mg/kg, i.p.) or 0.9% saline (1 ml/kg; i.p.) 45-55 min before the testing session to determine whether CNO itself had effect on prolonged freezing. Freezing was quantified by VideoFreeze software (Med Associates) based on a threshold of change in video image pixels. Rats that did not freeze at least 50% of the time for at least one of the 1 min bins in the training session were considered nonlearners and were not included in the analysis (N = 9).
In vivo single-unit extracellular electrophysiology
A separate cohort of rats was anesthetized with urethane (1.5 g/kg i.p.; Sigma Aldrich) and secured into a stereotaxic device (David Kopf Instruments). Proper plane of anesthesia was monitored via periodic hindlimb pedal reflex testing. Body temperature was recorded with a rectal temperature probe and maintained at 37°C with a heating pad (DC Temperature Controller, FHC). Before surgery, 1% lidocaine was injected at the incision site (0.2 ml, Webster Veterinary Supply) before the skull was exposed and burr holes were drilled above the BLA and BNST. Antidromic identification of BLA-BNST neurons used a concentric bipolar stimulation electrode (Microprobes) lowered into the BNST (1.5-1.6 mm lateral, 0 mm caudal, and 6.9 mm ventral from bregma) to deliver electrical stimulation (S48 Square Plus Stimulator, Grass Instruments). Single-barrel glass electrodes (borosilicate 2.0 mm o.d., World Precision Instruments) were heat-pulled using a vertical microelectrode puller (PE-2; Narishige) and broken under a light microscope to a tip diameter of 1-2 μm. Filled recording electrodes (2% Pontamine Sky Blue in 2 m NaCl) were slowly lowered into the BLA (−4.8 to −5.6 mm lateral, −2.8 to −3.8 mm caudal, and 6.5-9.0 mm ventral from bregma) using a hydraulic micromanipulator (Model MO-10, Narishige). Electrophysiological recordings did not begin until at least 1 h after electrode placements. Electrophysiological signals from the recording electrode were amplified (ER-1 Differential Extracellular Amplifier, Cygnus Technology), filtered at 0.1 Hz (low frequency) and 10 kHz (high frequency), and then digitized (InstruTECH ITC-18, HEKA Elektronik) and captured with Axograph X software on a Mac Pro Computer (Apple) for subsequent analysis. Signals were also monitored audibly (AM 10 Audio Monitor). To mark electrode placements on conclusion of experiments, Pontamine Sky Blue was ejected from the recording electrode (−30.0 μA, Constant Current Source, Finntronics) for at least 30 min, and the placement of the stimulation electrode was marked by iron deposits by applying three stimulation pulses (1 mA, 10 s duration) as previously described (Blume et al., 2017). Rats were then decapitated, and the brains removed and stored overnight in 4% PFA with 0.05% potassium ferrocyanide in 0.1 m PB and stored in 0.1 m PB until sectioning. Brains were sectioned (60 μm) using a vibratome (Leica Microsystems), then processed for Nissl staining. Placements were verified by light microscopy and reconstructed using a rat brain atlas (Paxinos and Watson, 2007).
Whole-cell ex vivo electrophysiology
Whole-cell recordings were obtained from visually identified neurons of the BLA under infrared differential interference contrast conditions as described previously (Hetzel et al., 2012). Animals (N = 14 males, 15 females) were anesthetized with ketamine (90 mg/kg, i.p., Ketaved) and xylazine (10 mg/kg; Anased, Webster Veterinary Supply) and transcardially perfused with ice-cold high-sucrose ACSF containing the following (in mm): 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 7 dextrose, 7 MgCl2, 0.5 CaCl2, 210 sucrose, 1.3 ascorbic acid, and 3 sodium pyruvate (saturated with 95% O2/5% CO2). Osmolality of high sucrose ACSF was ∼290 mOsm. The brain was rapidly removed and sectioned at 300 µm in a vibratome (Ted Pella) in ice-cold high sucrose ACSF, the brain slices recovered for ∼1 h at 34°C in physiological ACSF containing the following (in mm): 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 10 dextrose, 1 MgCl2, and 2 CaCl2, with the addition of 1.3 mm ascorbic acid and 3 mm sodium pyruvate. Recordings were performed at 30°C-34°C in submerged slices in physiological extracellular ACSF. (+)-Bicuculline (10 μm; Ascent Scientific; dissolved in DMSO), picrotoxin (10 μm; dissolved in ethanol), CNQX disodium salt (10 μm; Ascent Scientific, dissolved in ddH2O), and DL-AP5 sodium salt (50 μm; Ascent Scientific dissolved in 100 mm NaOH) were added to the ACSF for all experiments to block GABAA, AMPA, and NMDA receptor-mediated currents. Final solvent concentrations were <0.1% of the total ACSF volume. Solutions were saturated with 95% O2/5% CO2. Electrodes (1.8-9.5 mΩ open tip resistance) were filled with an intracellular solution containing the following (in mm): 120 K-gluconate, 20 KCl, 0.2 EGTA, 10 HEPES, 2 NaCl, 4 ATP-Mg, 0.3 GTP-Tris, 7 Tris-phosphocreatine, and 0.2% neurobiotin (to visualize recorded neurons; Vector Laboratories), with a pH of 7.3.
Whole-cell recordings were performed in bridge mode from visually identified pyramidal neurons within the BLA (AxoClamp 2B, Molecular Devices). Signals were low-pass filtered at 3-5 kHz and digitalized at 10 kHz (ITC-18, Heka Instruments). Mean series resistance for each group was <25 mΩ. All electrophysiology data were monitored with AxoGraph X software (Axograph Scientific) and stored on a computer (Mac Pro, Apple) for offline analysis. The resting membrane potential (Vrest) of each cell was recorded. Cells with a Vrest >−60 mV, which typically had electrophysiological features of interneurons, or were unhealthy principal neurons, were discarded. To measure excitability, depolarizing current steps were applied (0-250 pA, 50 pA increments, 1 s, repeated 3 times) from a membrane potential of −70 mV. The mean number of action potentials (APs) during each depolarization step was plotted against the amount of current injected. The medium and slow after-hyperpolarizations (mAHPs and sAHPs) were measured as the response to a train of 5 depolarizing current pulses (10 ms at 700 pA each, evoking a train of 5 APs) at 50 Hz, from a membrane potential of −55 mV. Only AHPs elicited by 1 AP/10 ms depolarization pulse were used for analysis. The train was repeated 10 times (10-20 s intervals), and the average AHP response was measured. The mAHP (at AHP peak) and the sAHP (270-300 ms) amplitudes were analyzed separately. In a similar manner, an AHP current (IAHP) was measured in voltage clamp from −50 mV after a step to 10 mV (500 ms). The AHP amplitude was calculated by subtraction from the prestep baseline.
In order to perform ex vivo electrophysiological recordings on identified BLA-BNST neurons, a subset of rats (N = 3 males, 3 females) were injected bilaterally with retrobeads (500 nl; Lumafluor) into the BNST (±1.5-1.6 mm lateral, 0 mm caudal, and 6.9 mm ventral from bregma) using the same surgical procedure as described above. Rats were then allowed to recover for at least 10 d to allow the retrograde transport of the fluorescent beads before ex vivo electrophysiological recordings. Injection sites in the BNST were visually confirmed during coronal sectioning of tissue. Recordings were obtained from neurons that displayed fluorescence in response to blue light (peak excitation 460 nm, peak emission 505; GFP filter set) and adjacent neurons that did not contain beads.
To validate the effectiveness of DREADD in neurons from male and female rats, ex vivo whole-cell recordings were performed 21 d following viral surgery, as described above. DREADD+ neurons were identified based on mCherry fluorescence (peak excitation 560, peak emission 630, mCherry filter set). Upon successful stable recording, baseline measures of excitability and input resistance were obtained. CNO was applied by bath (10 μm), and measures were obtained again after 10-15 min (N = 5 neurons from 2 female rats; N = 4 neurons from 1 male rat). Nonfluorescent neurons were similarly recorded, and the effects of bath-applied CNO on these neurons were tested as a control (N = 5 neurons from same 3 rats).
Histology
After recordings, slices were fixed in 4% PFA in 0.1 m PBS for up to 4 weeks at 4°C. Sections were rinsed 3 times with PBS, treated with Triton X-100 (VWR International; 1% in PBS) for 6-8 h, and then incubated in the Vectastain ABC Reagent (Vector Laboratories) in PBS at room temperature overnight. After three rinses with PBS, sections were reacted with DAB and H2O2 (Peroxidase Substrate Kit DAB, Vector Laboratories) in water to visualize the neurobiotin-filled neurons. Sections were washed in PBS repeatedly to stop the reaction. Sections were mounted, dried, and coverslips were applied. Stained sections were used to localize the recording sites, verified by the position of the filled neurons. Neurons were furthered classified as BLA principal neurons by their position and morphologic characteristics that were consistent with pyramidal neurons (McDonald, 1982, 1984).
Experimental design and statistical analyses
All behavioral experiments were performed on intervals of 4 d to measure at the same stage of estrus in females. Statistical analysis was performed using Prism 8 software (GraphPad Software). Data were compared between males and females (metestrus and proestrus combined) to test whether there are sex differences, or between metestrous and proestrous females to determine whether there are cycle stage-dependent differences. Data were also compared by drug treatment (CNO vs saline) in both male and female rats combined to determine whether there were significant differences in response to BLA-BNST inhibition. Data with two groups were compared with an unpaired Student's t test, and more than two groups were analyzed with a one-way ANOVA. When comparing across more than one factor (e.g., drug and sex), two-way ANOVA or two way repeated-measures (RM) ANOVA was performed. For all two-way RM ANOVA tests, sphericity was not assumed and a Geisser–Greenhouse correction was performed. Holm–Sidak's multiple comparisons test was used for further post hoc analysis if appropriate. Significance was set at p < 0.05. All graphed data are presented as mean ± SEM. Outlier data points were removed if they were >2 SDs from the mean (N = 2).
Results
Female rats extinguish sustained fear more rapidly than males
We first validated a sustained fear model (Fig. 1A) that paired a prolonged CS with random footshocks (Waddell et al., 2006) in naive male and female rats. Footshock intensity was determined from a separate group of rats (N = 3 male, N = 6 females) using varying intensities delivered through the floor grid in 0.02 mA increments from 0.1 mA (0.1, 0.12, 0.14, etc.) until a consistent forepaw withdrawal with an avoidance response (e.g., backpedaling) was noted. Based on the analysis of this response, a footshock intensity of 0.3 mA was determined to be appropriate for all of the conditioning chambers.
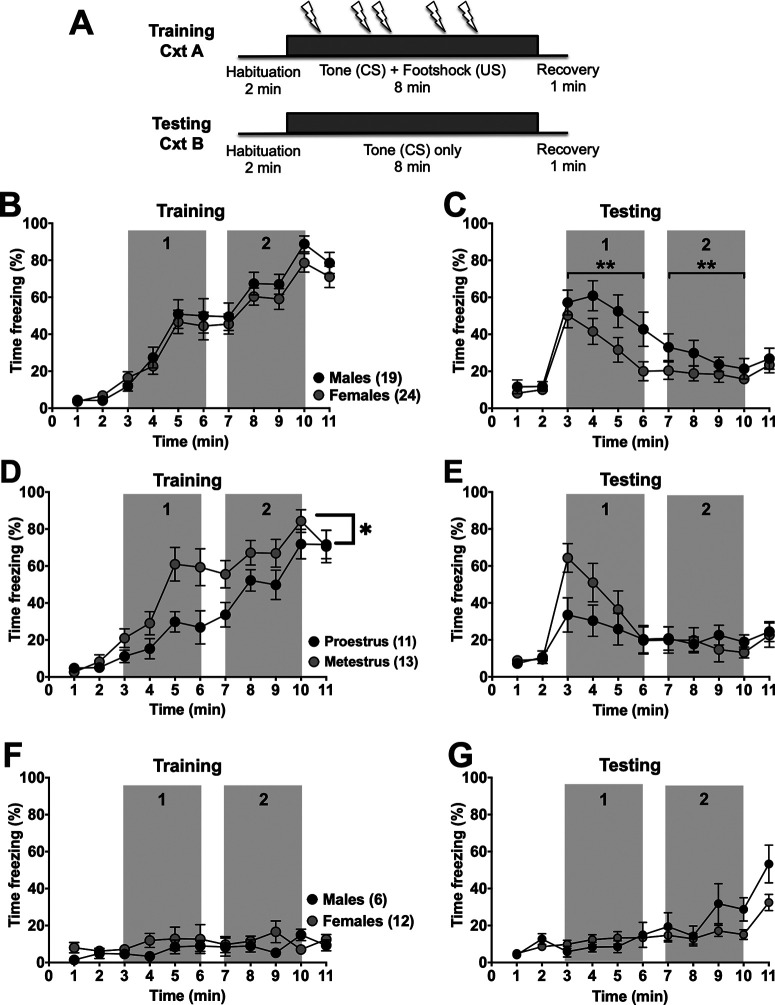
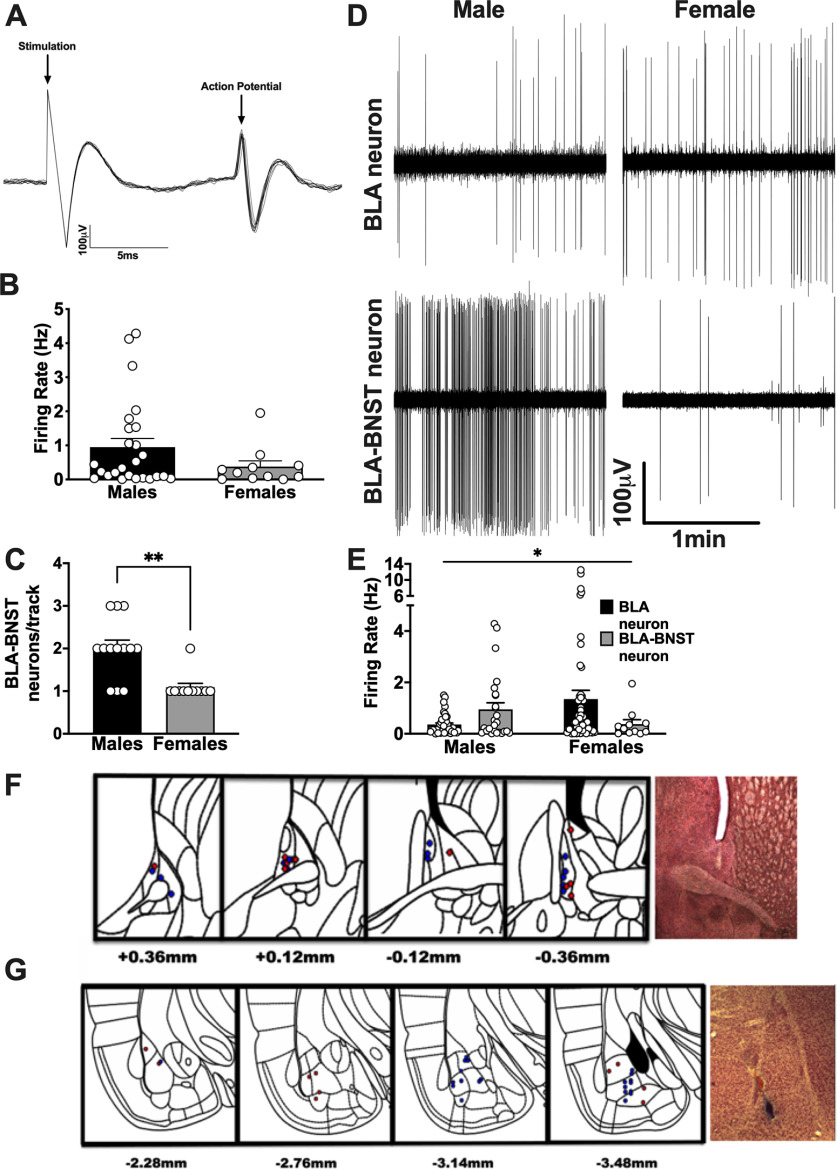
Figure 1.
Prolonged cued-conditioned freezing is lower in females compared with males. A, Schematic representation of the prolonged cued fear paradigm where rats are conditioned to the prolonged tone (CS; 5 kHz, 80 dB, 8 min; gray boxes) and receive 5 randomized foot shocks (0.3 mA, 0.5 ms) in context A (Cxt A). Four days later in a separate context (Cxt B), percent time freezing during the CS in the absence of footshocks was tested. B, Percent time spent freezing (1 min bin averages) in naive male (black, N = 19) and female (gray, N = 24) rats during prolonged cued fear conditioning was analyzed, but there was only a significant main effect of time (F(3.705,151.9) = 74.27, p < 0.0001, two-way RM ANOVA) but no main effect of sex (F(1,41) = 0.8288, p = 0.3679, two-way RM ANOVA) nor an interaction between sex and time (F(10,410) = 0.5015, p = 0.8889, two-way RM ANOVA). There were no differences in the total time spent freezing for the first 4 min of the tone (1; t(41) = 1.190, p = 0.21, unpaired t test) and the last 4 min of the tone (2; t(41) = 0.334, p =0.740, unpaired t test) between males and females during prolonged cued fear training. C, During testing, there was a main effect of time (F(3.657,150) = 24.31, p < 0.0001), and a trend for a main effect of sex (F(1,41) = 3.497, p = 0.0687, two-way RM ANOVA), but no significant interaction between time and sex (F(10,410) = 1.613, p = 0.1004, two-way RM ANOVA). Females had significantly lower total time spent freezing during both the first half of the tone (1; t(41) = 3.903, **p = 0.0003, unpaired t test) and second half of the tone (2; t(41) = 5.424, **p < 0.0001, unpaired t test) compared with males during prolonged cued fear testing. Percent time freezing was also calculated and compared by estrous cycle stage (black, N = 11 proestrous; and gray, N = 13 metestrous) and time (D). There were main effects of both time (F(4.376,96.27) = 38.09, p < 0.0001, two-way RM ANOVA) and estrous cycle stage (F(1,22) = 7.127, p = 0.014, two-way RM ANOVA), with all animals spending more time freezing as the experiment progressed, and proestrous females freezing less overall compared with metestrous females. There was also a significant interaction between time and estrous cycle stage (F(10,220) = 1.947, *p = 0.0404, two-way RM ANOVA). E, A significant interaction between time and estrous cycle phase was found during testing (F(10,220) = 2.573, p = 0.0058, two-way RM ANOVA) as proestrous rats froze significantly less than metestrous females during testing. There was also a main effect of time (F(10,220) = 11.44, p < 0.0001, two-way RM ANOVA), but not cycle stage (F(1,22) = 0.4649, p = 0.5024, two-way RM ANOVA) as both groups reduced freezing over time. F, A separate group of male (black, N = 6) and female (gray, N = 12) rats were conditioned to the tone in the absence of footshock. There was no main effect of time (F(3.311,33.11) = 1.224, p = 0.3177, two-way RM ANOVA), sex (F(1,10) = 1.317, p = 0.2779, two-way RM ANOVA), nor interaction between the two (F(10,100) = 1.224, p = 0.2853, two-way RM ANOVA) during training. G, These same rats were then tested in Cxt B to ensure that there were no sex differences in freezing in response to tone alone. While there was no main effect of sex (F(1,16) = 2.274, p = 0.151, two-way RM ANOVA), we did have a main effect of time (F(4.294,68.71) = 15.72, p < 0.0001, two-way RM ANOVA) as rats began to freeze at the end of the experiment. Furthermore, there was also a significant interaction (F(10,160) = 2.548, p = 0.007, two-way RM ANOVA) between time and sex as male rats froze near the end of the experiment more than females.
Throughout all experiments, estrous cyclicity was monitored in female rats, and there was a 4 d interval between training and testing for all groups so that female rats would be in the same estrous phase during training and testing sessions. During the training session, both male and female rats exhibited similar increases in freezing (Fig. 1B). During the testing session, female rats extinguished freezing behavior more rapidly than males (Fig. 1C), despite similar freezing during conditioning. Data were analyzed across time to provide insight into whether initial expression (first half; Fig. 1C) or subsequent extinction (second half; Fig. 1C) was selectively different. Indeed, during the testing session, females spent significantly less time freezing during the first half of the tone (gray box 1; 35.85 ± 17.73 s vs 128.00 ± 5.69 s, t(41) = 5.424, p < 0.0001) as well as the second half of the tone (gray box 2; 18.33 ± 12.73 s vs 64.76 ± 0.33 s, t(41) = 3.903, p = 0.0003) than males. Along with similar freezing during conditioning, this suggests that the primary sex difference may be in the prolonged expression or acquisition of extinction. A similar number of male (N = 2) and female (N = 3) rats were poor learners, meaning they did not freeze (at least 50% of the time for 1 min) during the training session and therefore were not included in the analysis. Animals (males: N = 6; females: N = 12) that underwent training sessions in the absence of footshocks (Fig. 1F) and subsequent testing (Fig. 1G) did not freeze in the presence of a prolonged tone alone, indicating that the tone itself did not induce an aversive response. Moreover, rats did not freeze during the 2 min baseline before CS presentation during the extinction testing session in a novel context, suggesting that freezing was specific to the prolonged tone and not poor discrimination of the context. Together, these experiments confirmed the effectiveness of this sustained fear model and indicated that sex differences exist during acquisition of extinction of a sustained anticipatory conditioned response.
BLA-BNST inactivation reduces prolonged conditioned freezing in male, but not female, rats
To determine whether BLA inputs into the BNST mediate conditioned freezing during prolonged cues, we used an intersectional chemogenetic approach to inhibit BLA-BNST neurons. Injecting a retrograde CAV2-Cre-recombinase into the BNST and an inhibitory Cre-recombinase-dependent DREADD-Gi into the BLA of male and female rats (Fig. 2A,B) enabled temporary inhibition of this pathway with administration of CNO (1 mg/kg, i.p.) as confirmed with ex vivo electrophysiology (Fig. 2C,D). After allowing at least 21 d for virus transfection, prolonged cued fear training was performed as described (see Materials and Methods). Four days later, either 0.9% saline (1 ml/kg, i.p.) or CNO (1 mg/kg, i.p.) was injected before the testing session to inhibit BLA-BNST neurons during fear expression and acquisition of fear extinction (Fig. 3A). There were no significant differences in freezing between rats (males and females combined) in either the CNO or control groups during prolonged cued fear training (Fig. 3B) nor during testing session after either saline or CNO injections (Fig. 3C). Since our previous studies in naive rats showed sex differences in prolonged cued fear responses, we also analyzed BLA-BNST inhibition within sexes. Indeed, we found that, while freezing during training was similar between groups (Fig. 3D,F), sex differences emerged during prolonged cued fear extinction (Fig. 3E,G). Specifically, BLA-BNST inhibition by injection of CNO in males reduced conditioned freezing during the first half of the tone compared with control males (Fig. 3H–K; gray box 1; 48.69 ± 10.34 s vs 98.23 ± 29.03 s), whereas BLA-BNST inhibition increased conditioned freezing behavior in females compared with controls during the same time period (gray box 1; 108.40 ± 20.96 s vs 71.77 ± 16.75 s) with a main interaction between sex and drug (F(1,39) = 4.636, p = 0.0375). These data suggest that inhibition of the BLA-BNST pathway reduces expression of prolonged conditioned freezing during extinction in males, but not females, and indicates that this pathway is activated during these behaviors in males. To test whether CNO administration during extinction had alternative actions that might impact fear-related behavior, we also quantified freezing behavior in a group of naive rats (N = 10 saline and N = 12 CNO) that did not undergo viral-injection surgeries (Fig. 4). CNO administration did not produce any significant differences in freezing behavior within either sex during the training or testing sessions, which suggests that CNO administration alone does not reduce freezing during extinction to a prolonged cue.
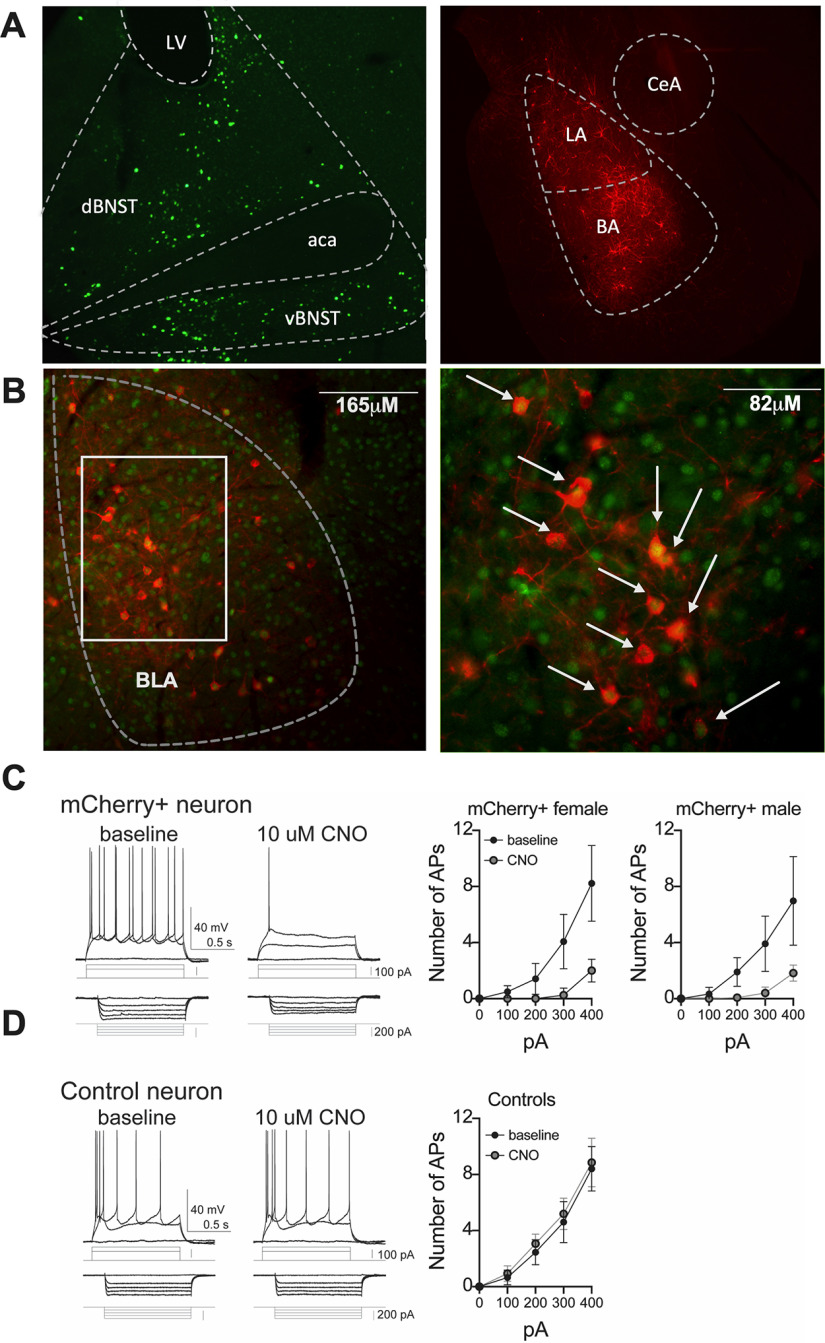
Figure 2.
Intersectional chemogenetic targeting of BLA-BNST neurons with Cre-dependent DREADDs. A, CAV2-Cre was injected bilaterally into the BNST (top left) in combination with Cre-dependent inhibitory DREADDs in the BLA (top right). Photomicrograph illustrating Cre-recombinase (bottom left; green) and mCherry (bottom right; red) immunoreactivity in the BLA of virus-injected rats. B, Photomicrograph examples of Cre-recombinase (green) and mCherry (red) coexpression in the BLA at low (top) and high magnification (bottom). White arrows indicate double-labeled cells. C, DREADD+ neurons were identified for ex vivo recordings based on mCherry fluorescence. CNO (bath-applied, 10 μm) significantly decreased excitability of DREADD+ neurons from females (N = 5 neurons, p < 0.001, F(1,20) = 70.4, two-way RM ANOVA) and males (N = 4 neurons, p < 0.01, F(1,15) = 40.6, two-way RM ANOVA). D, In contrast, CNO did not significantly impact the excitability of non-DREADD+ neurons (N = 5 neurons, p > 0.05, F(1,20) = 0.31, two-way RM ANOVA).
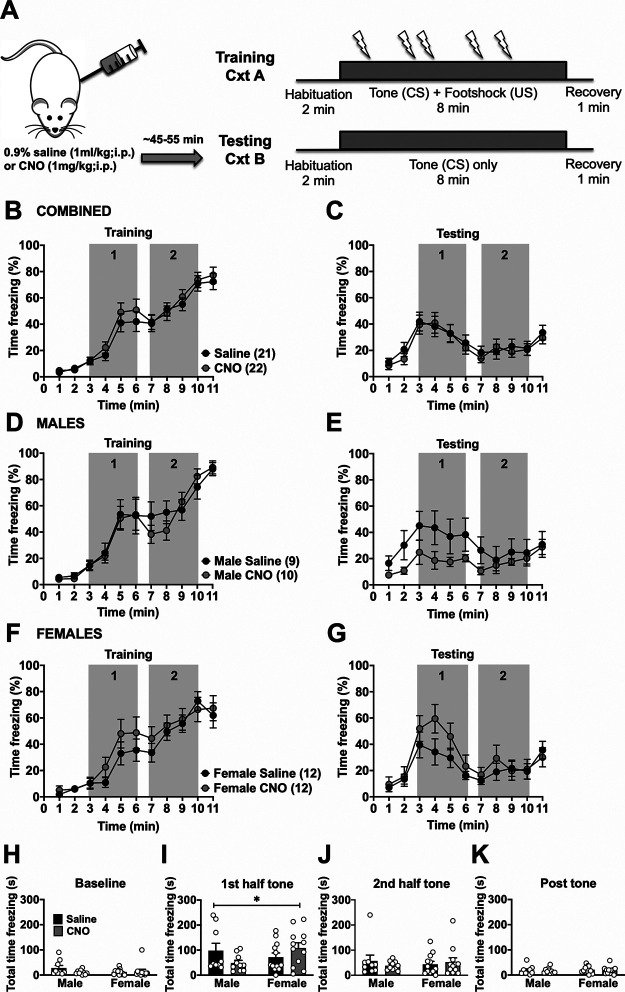
Figure 3.
BLA-BNST inactivation has opposing effects on freezing to a prolonged conditioned cue in male and female rats. A, Rats were conditioned as described previously (Fig. 1A), then injected with either saline (N = 21, black circles) or CNO (N = 22, gray circles) before testing. B, There was only a main effect of time (F(4.557,186.8) = 81.06, p < 0.0001, two-way RM ANOVA) during prolonged cued fear training. There was no significant main effect of CNO treatment (F(1,41) = 0.3874, p = 0.5371, two-way RM ANOVA), nor was there a main interaction between time and CNO (F(10,410) = 0.4609, p = 0.9146, two-way RM ANOVA). C, During extinction, when all rats were grouped together, there was only a main effect of time (F(2.9868,121.7) = 12.31, p < 0.0001, two-way RM ANOVA) on freezing behavior, but no main effect of drug alone (F(1,41) = 0.1296, p = 0.7207, two-way RM ANOVA) nor interaction between drug and time (F(10,410) = 0.3397, p = 0.9699, two-way RM ANOVA). D, For male rats that received either saline (N = 9, black circles) or CNO (N = 10, gray circles) injections before testing, there was only a main effect of time (F(3.33,56.61) = 46.98, p < 0.0001, two-way RM ANOVA) on freezing during training, but no main drug effect (F(1,17) = 0.06733, p = 0.7984, two-way RM ANOVA) nor significant interaction (F(10,170) = 0.7074, p = 0.7167, two-way RM ANOVA). E, During testing, there was only a main effect of time (F(2.802,47.63) = 3.528, p = 0.024, two-way RM ANOVA), but not of drug (F(1,17) = 2.176, p = 0.1584, two-way RM ANOVA) nor an interaction (F(10,170) = 1.143, p = 0.3331, two-way RM ANOVA). F, Female rats that were injected with either saline (N = 12, black circles) or CNO (N = 12, gray circles) had a main effect of time on freezing during training (F(4.794 105.5) = 38.18, p < 0.0001, two-way RM ANOVA), but not for drug (F(1,22) = 0.8739, p = 0.36, two-way RM ANOVA) nor an interaction between drug and time (F(10,220) = 0.7041, p = 0.7201, two-way RM ANOVA). G, Similarly, during testing sessions, there was only a main effect of time (F(2.868,63.10) = 10.75, p < 0.0001, two-way RM ANOVA), but not drug alone (F(1,22) = 0.8374, p = 0.3701, two-way RM ANOVA) nor an interaction between time and drug (F(10,220) = 1.239, p = 0.2673, two-way RM ANOVA). H, Total time spent freezing during 2 min baseline, the first 4 min of the prolonged tone (I), the second 4 min of the prolonged tone (J), and the postshock recovery period (K) of the testing session were separately analyzed. There was a significant interaction between drug and sex during the first half of the tone (H; F(1,39) = 4.636, *p = 0.0375, two-way ANOVA) as CNO treatment reduced freezing in males, but increased freezing in females during the first half of the tone. There was no main effect of drug (F(1,39) = 0.1044, p = 0.7483, two-way ANOVA) or sex (F(1,39) = 0.6896, p = 0.4114, two-way ANOVA) alone.
Figure 4.
CNO administration during prolonged fear extinction does not alter freezing behavior in naive rats. A, Naive rats were conditioned as described previously (Fig. 1A). Rats were injected with either saline (N = 10; black circles) or CNO (N = 12; gray circles) before extinction. There was only a main effect of time on freezing behavior during training (B; F(2.489,22.4) = 34.74, p < 0.0001, two-way RM ANOVA) and testing (C; F(3.291,29.62) = 7.985, p = 0.0003, two-way RM ANOVA) in males. There was no significant effect of drug (F(1,9) = 0.08397, p = 0.7786, two-way RM ANOVA) nor interaction between time and drug (F(10,90) = 0.6511, p = 0.7661, two-way RM ANOVA) during training. The lack of effect of drug (F(1,9) = 0.3623, p = 0.5621, two-way RM ANOVA) or interaction (F(10,90) = 0.6585, p = 0.7596, two-way RM ANOVA) was also observed during testing. Similar results were observed in females with only a main effect of time during both training (D; F(3.74,33.66) = 31.44, p < 0.0001, two-way RM ANOVA) and testing (E; F(3.631,32.68) = 7.177, p = 0.0004, two-way RM ANOVA). Females also did not have a main effect of drug (F(1,9) = 0.0623, p = 0.8085, two-way RM ANOVA) nor interaction (F(10,90) = 0.8144, p = 0.6155, two-way ANOVA) during training and testing (main effect of drug; F(1,9) = 0.02609, p = 0.8752, two-way RM ANOVA; main interaction; F(10,90) = 0.2640, p = 0.9873, two-way RM ANOVA).
To determine whether BLA-BNST is involved with other aspects of fear conditioning, we also injected CNO to suppress BLA-BNST neuronal activity during prolonged cued fear training (Fig. 5A). We found no differences in sustained fear learning between the saline- and CNO-treated groups (Fig. 5B,C). Even when separated by sex, no differences in freezing behaviors during conditioning or testing were observed after CNO treatment in males (Fig. 5D,E) or females (Fig. 5F,G). This suggests that BLA-BNST neurons do not have an active role during sustained fear learning, rather they specifically mediate freezing behavior during prolonged cued fear expression. To examine this further, a correlational analysis was performed to test whether animals that froze more during conditioning also froze more during testing. There was a moderate correlation between freezing during training and freezing during testing in both sexes, although this only reached significance in females (males, correlation coefficient r(17) = 0.4028, p = 0.0873; females, correlation coefficient r(22) = 0.6504, p = 0.0006). This suggests that this relatively small effect of CNO during training in females may be related to differences in individual animals (e.g., estrous phase, examined below).
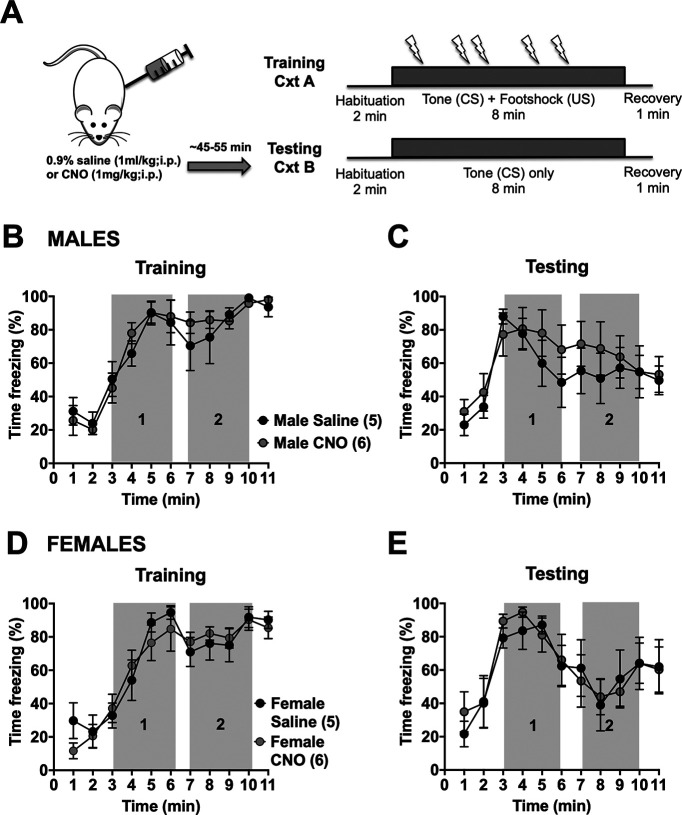
Figure 5.
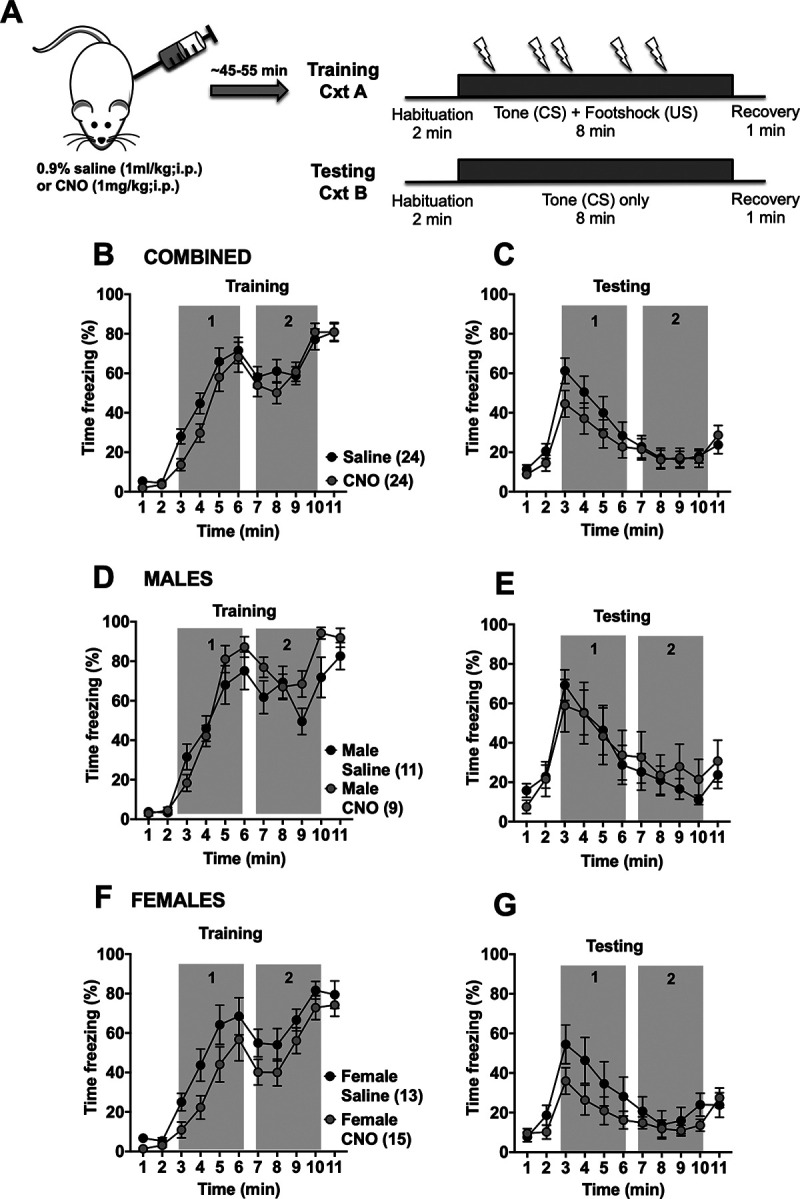
Inactivation of BLA-BNST neurons during conditioning does not affect sustained freezing to a prolonged cue. A, Rats were conditioned as described previously (Fig. 1A). B, Rats were injected with either saline (N = 24; black circles) or CNO (N = 24; gray circles) before conditioning. There was a main effect of time (B; F(4.46,205.2) = 104, p < 0.0001, two-way RM ANOVA) on freezing behavior during training and testing (C; F(3.489,160.5) = 19.95, p < 0.0001, two-way RM ANOVA). There was no main effect of drug (F(1,46) = 1.144, p = 0.2904, two-way RM ANOVA) nor interaction (F(10,460) = 1.356, p = 0.1983, two-way RM ANOVA) during training or testing (main effect of drug, F(1,46) = 0.7682, p = 0.3853, two-way RM ANOVA; main interaction, F(10,460) = 1.245, p = 0.2596, two-way RM ANOVA). When animals were separated out by sex, BLA-BNST inhibition during prolonged cued fear training had only a main effect of time during both training (D; F(4.243,93.35) = 57.8, p < 0.0001, two-way RM ANOVA) and testing (E; F(3.761,82.74) = 13.48, p < 0.0001, two-way RM ANOVA) in males. No main effect of drug nor interaction between drug and time was observed during training (main effect of drug, F(1,22) = 0.1057, p = 0.7482, two-way RM ANOVA; main interaction, F(10,220) = 1.028, p = 0.4205, two-way RM ANOVA) nor testing (main effect of drug, F(1,22) = 0.002366, p = 0.9616, two-way RM ANOVA; main interaction, F(10,220) = 0.5052, p = 0.8854, two-way RM ANOVA) in males. Similar results were observed in females with only a main effect of time during both training (F; F(3.82,99.32) = 53.38, p < 0.0001, two-way RM ANOVA) and testing (G; F(3.255,84.63) = 9.072, p < 0.0001, two-way RM ANOVA) but no main effect of drug nor interaction during training (main effect of drug, F(1,26) = 3.528, p = 0.0716, two-way RM ANOVA; main interaction, F(10,260) = 0.7071, p = 0.7176, two-way RM ANOVA) or testing (main effect of drug, F(1,26) = 2.010, p = 0.1682, two-way RM ANOVA; main interaction, F(10,260) = 1.105, p = 0.3589, two-way RM ANOVA).
BLA-BNST inactivation does not affect OF exploration
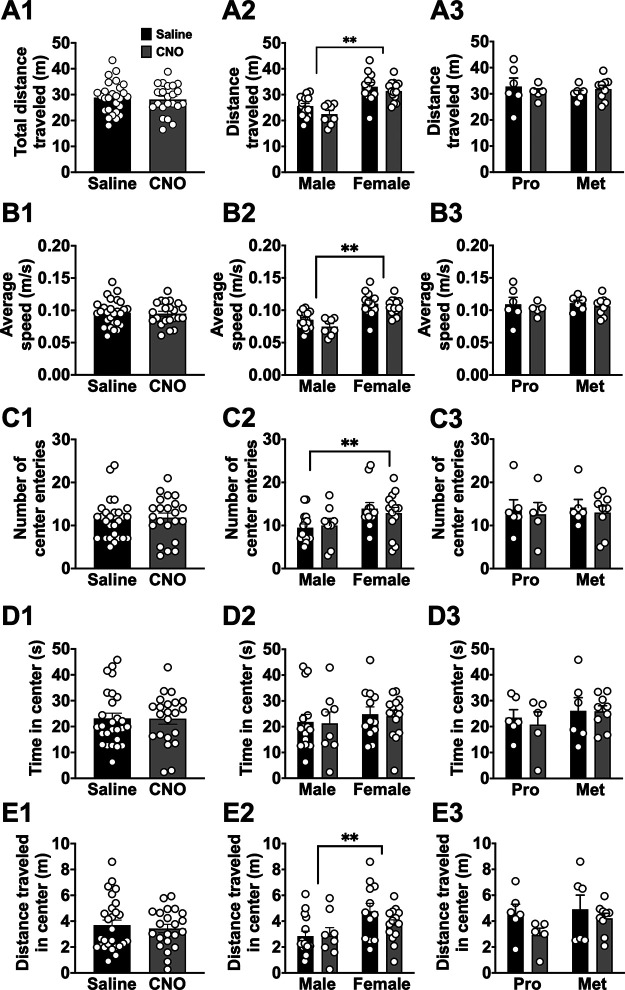
We next determined whether BLA-BNST circuitry is necessary for expression of other behaviors sensitive to defensive states and anxiety, such as open field (OF) exploration and SI. Virus-treated rats were randomly assigned to receive either 0.9% saline (1 ml/kg; i.p.) or CNO (1 mg/kg; i.p.) injections 45-55 min before the OF task. Inactivation of BLA-BNST neurons had no effect on any parameters of OF exploration (Fig. 6). When we separated animals out by sex, a main effect of sex was observed: females traveled a significantly greater distance in the center area (Fig. 6E2; F(1,45) = 7.997, **p = 0.007, two-way ANOVA), made more center entries (Fig. 6C2; F(1,45) = 7.703, **p = 0.008, two-way ANOVA), and had a significantly higher average speed (Fig. 6B2; F(1,45) = 39.37, **p < 0.0001, two-way ANOVA) and total distance traveled (Fig. 6A2; F(1,45) = 39.56, p < 0.0001, two-way ANOVA) compared with males. There was no effect of CNO treatment (BLA-BNST inhibition) on any of the parameters in either sex. This indicates that BLA-BNST pathway may not be engaged during OF exploration in either sex.
Figure 6.
OF exploration is not affected by inactivation of BLA-BNST. OF exploration was examined in rats following either CNO (N = 22, gray) or saline (N = 27, black) injections. There were no significant differences in total distance traveled (A1; t(47) = 0.4362, p = 0.6647, unpaired t test), average speed (B1; t(47) = 0.2639, p = 0.7930, unpaired t test), number of center entries (C1; t(47) = 0.2722, p = 0.7866, unpaired t test), time spent in the center (D1; t(47) = 0.03786, p = 0.97, unpaired t test), and distance traveled in the center (E1; t(47) = 0.5061, p = 0.6152, unpaired t test) during the novel OF task. The same parameters were also analyzed by sex and drug treatment (N = 15 saline males, N = 8 CNO males, N = 12 saline females, and N = 14 CNO females). There were main effects of sex when total distance traveled (A2; F(1,45) = 39.56, p < 0.0001, two-way ANOVA), average speed (B2; F(1,45) = 39.37, **p < 0.0001, two-way ANOVA), number of center entries (C2; F(1,45) = 7.703, **p = 0.008, two-way ANOVA), and distance traveled in the center (E2; F(1,45) = 7.997, **p = 0.007, two-way ANOVA) were analyzed. All other effects and interactions were not significant when total distance traveled (A2; main interaction, F(1,45) = 0.3066, p = 0.5825; main effect of drug, F(1,45) = 3.324, p = 0.0749, two-way ANOVA), average speed (B2; main interaction, F(1,45) = 0.3327, p = 0.5669; main effect of drug, F(1,45) = 3.327, p = 0.0748, two-way ANOVA), number of center entries (C2; main interaction, F(1,45) = 0.366, p = 0.5482; main effect of drug, F(1,45) = 0.03995, p = 0.8425, two-way ANOVA), time spent in the center (D2; main interaction, F(1,45) = 0.0009681, p = 0.9753; main effect sex, F(1,45) = 0.8749, p = 0.3546; main effect of drug, F(1,45) = 0.04559, p = 0.8319, two-way ANOVA), and distance traveled in the center (E2; main interaction, F(1,45) = 1.1, p = 0.2998; main effect of drug, F(1,45) = 0.9853, p = 0.3262, two-way ANOVA) were calculated. The times spent in the center (D2) were not significantly different between sex or by drug treatment. The same parameters were also analyzed by estrous cycle stage and drug treatment (proestrous, N = 11; and metestrous, N = 15). Total distance traveled (A3; main effect cycle stage, F(1,22) = 0.111, p = 0.7421; main effect of drug, F(1,22) = 0.028, p = 0.8686; main interaction, F(1,22) = 0.9335, p = 0.3445, two-way ANOVA), average speed (B3; main interaction, F(1,22) = 0.03899, p = 0.8453; main effect cycle, F(1,22) = 0.1992, p = 0.6597; main effect of drug, F(1,22) = 0.8147, p = 0.3765, two-way ANOVA), number of center entries (C3; main interaction, F(1,22) = 0.0005892, p = 0.9809; main effect of cycle, F(1,22) = 0.04773, p = 0.8291; main effect of drug, F(1,22) = 0.2939, p = 0.5932, two-way ANOVA), time in the center (D3; main interaction, F(1,22) = 0.113, p = 0.7399; main effect of cycle, F(1,22) = 1.088, p = 0.3083; main effect of drug, F(1,22) = 0.1594, p = 0.6936, two-way ANOVA), and distance traveled in the center (E3; main interaction, F(1,22) = 0.4916, p = 0.4905; main effect of cycle, F(1,22) = 1.298, p = 0.2668; main effect of drug, F(1,22) = 2.875, p = 0.1041, two-way ANOVA) were calculated and determined to be comparable between all groups.
BLA-BNST inhibition increases social interaction in male, but not female, rats
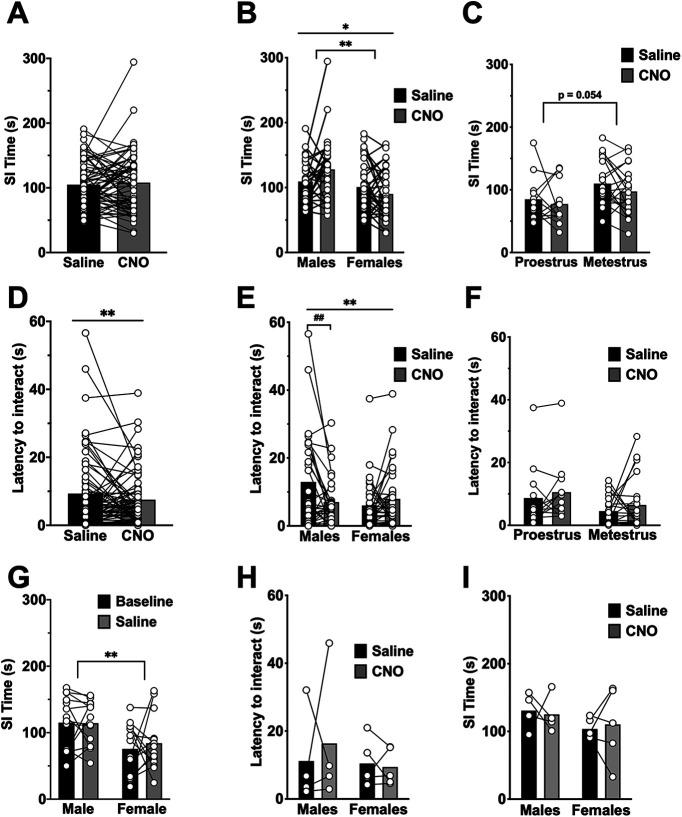
SI behavior involves the BLA and BNST, and differs between males and females (Johnston and File, 1991; Stack et al., 2010). Therefore, we also assessed the effect of BLA-BNST inhibition on potential changes to SI. All rats were first exposed to the OF at least 1 d before SI to acclimate to the field. In a subset of rats (N = 28), baseline SI was determined before drug treatment and was compared with SI in saline-treated animals 4 d later (Fig. 7G). Injection of saline (45-55 min before SI) did not significantly affect SI behavior compared with baseline.
Figure 7.
Inhibition of BLA-BNST neurons increases social interaction in males, but not females. A, The total time spent interacting was compared within individual animals (N = 63) following both CNO (black) and saline (gray) injections administered before SI tests and found to be similar (t(62) = 0.5433, p = 0.5889, paired t test). B, Rats were separated out by sex and drug treatment, and analysis determined a main effect of sex (F(1,61) = 7.949, **p = 0.0065, two-way RM ANOVA) as males spent more time interacting than females, but no main effect of drug (F(1,61) = 0.4725, p = 0.4944, two-way RM ANOVA). There was also a significant interaction between sex and drug (F(1,61) = 6.484, *p = 0.0134, two-way RM ANOVA) as males interacted more following BLA-BNST inhibition, but females did not. C, The total time spent interacting was compared within individual metestrous (N = 21) and proestrous (N = 12) rats following both CNO (gray) and saline (black) injections trended toward metestrous females interacting more than proestrous females (main interaction, F(1,31) = 0.1113, p = 0.741; main effect of cycle, F(1,31) = 4.012, p = 0.054; main effect of drug, F(1,31) = 1.833, p = 0.1856, two-way RM ANOVA). D, Latency to interact was comparable between saline- and CNO-treated rats (t(62) = 1.326, p = 0.1897, paired t test). E, However, when animals were separated out by sex, the latency to interact did have a significant interaction between sex and drug (F(1,61) = 9.342, **p = 0.0033, two-way RM ANOVA) with males that received CNO having a shorter latency to interact compared with males that received saline (Sidak test for multiple comparisons, ##p = 0.0045, 95% CI (1.665, 10.210)). There was no main effect of sex (F(1,61) = 1.997, p = 0.1627, two-way RM ANOVA) or drug alone (F(1,61) = 2.422, p = 0.1248, two-way RM ANOVA). F, The latency to interact in female rats following either a saline or CNO injection was also not different between cycle stage (main interaction, F(1,31) = 0.001501, p = 0.9693; main effect of cycle, F(1,31) = 2.702, p = 0.1103; main effect of drug, F(1,31) = 1.537, p = 0.2244, two-way RM ANOVA). G, Baseline SI time was analyzed in a subset of male (N = 14) and female (N = 14) rats and compared 4 d later to SI time following saline treatment. The total time spent interacting during the baseline SI task (black bars) compared with total interaction following saline injection (gray bars) was found to be similar (main interaction, F(1,26) = 0.2521, p = 0.6198; main effect of drug, F(1,26) = 0.1795, p = 0.6753, two-way RM AVOVA), but there was a main effect of sex (F(1,26) = 9.680, **p = 0.0045, two-way RM ANOVA) as males interacted more than females. A subset of rats (N = 4 males and N = 4 females) had the same viral injection procedure but did not receive CAV2-Cre injections and therefore had nonfunctional DREADDs. When these animals were injected with CNO, there were no significant changes in latency to interact (H; main interaction, F(1,7) = 0.2847, p = 0.6101; main effect of sex, F(1,7) = 0.4408, p = 0.528; main effect of drug, F(1,7) = 0.1279, p = 0.7312, two-way RM ANOVA) nor SI time (I; main interaction, F(1,7) = 0.1315, p = 0.7276; main effect of sex, F(1,7) = 1.555, p = 0.2525; main effect of drug, F(1,7) = 0.001323, p = 0.972, two-way RM ANOVA).
Rats were injected with either 0.9% saline (1 ml/kg, i.p.) or CNO (1 mg/kg; i.p.) 45-55 min before the start of the SI test and placed into the OF where they interacted with a novel rat. Four days later, rats were injected with the opposite treatment (saline or CNO; 45-55 min) before a second SI test with a novel rat. This allowed for comparison of behavioral effects following saline versus CNO injections (BLA-BNST inhibition) within an individual animal. Inhibition of BLA-BNST by CNO treatment did not alter the total time spent interacting (Fig. 7A; paired t test, p > 0.05), nor did it significantly reduce the latency to interact with a novel rat (Fig. 7D; paired t test p > 0.05). When rats were analyzed by sex and treatment, SI time was significantly increased by CNO treatment in males (CNO: 127.82 ± 8.08 s vs saline: 109.2 ± 6.07 s), but not females (Fig. 7B; CNO: 89.98 ± 6.50 s vs saline: 100.68 ± 6.81 s; main effect of sex, F(1,61) = 7.949, **p = 0.0065, and interaction between sex and drug, F(1,61) = 6.484, *p = 0.0134). Furthermore, the latency to interact was significantly decreased in males following BLA-BNST inhibition, but not females (Fig. 7E; main interaction between drug and sex, F(1,61) = 9.342, **p = 0.0033). To determine whether CNO injection itself had an effect on SI behavior, CNO was given to a group of control rats (N = 8; AAV-DREADD-Gi but no CAV2-Cre injection). Administration of CNO did not alter latency to interact (Fig. 7H; paired t test, p > 0.05) or total SI (Fig. 7I; paired t test, p > 0.05) in either sex (N = 4/sex). Together, these data implicate the BLA-BNST pathway in the modulation of SI behavior in males, but not females.
BLA-BNST inhibition does not affect sustained fear across the estrous cycle
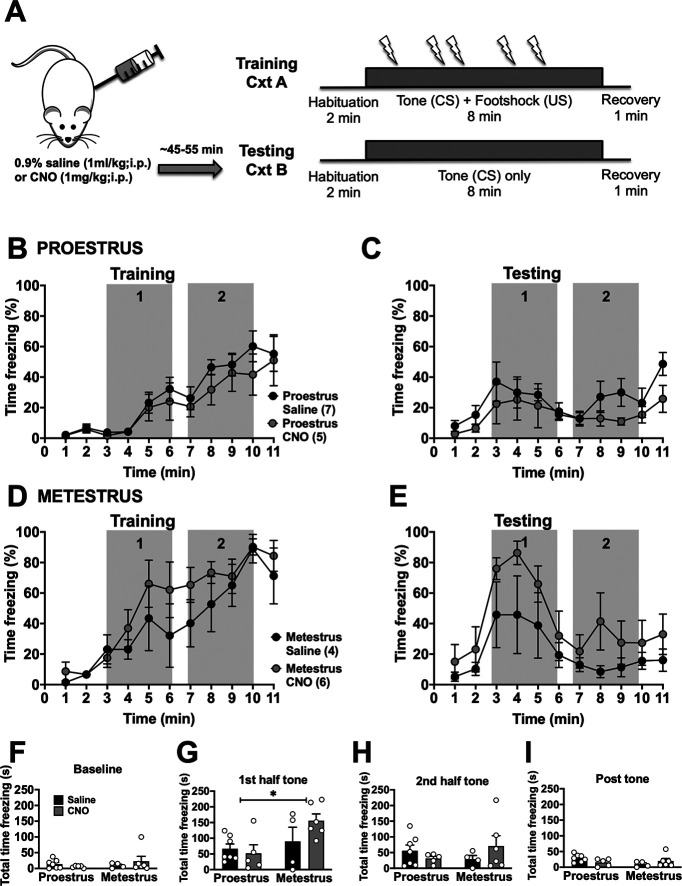
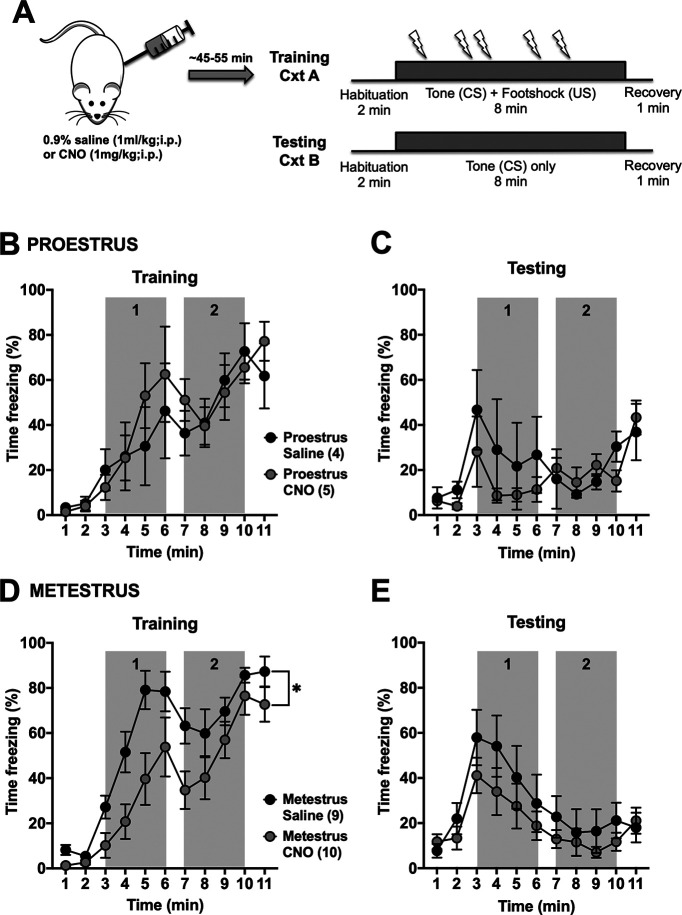
Our results suggest that the activity of the BLA-BNST pathway has a limited role on the expression of prolonged fear conditioning in females. Previous studies from our laboratory and others demonstrate that BLA activity and BLA-dependent behaviors vary across the estrous cycle (Blume et al., 2017, 2019; Lovick and Zangrossi, 2021). Therefore, we grouped our females by estrous cycle stage in a separate analysis. During prolonged cued fear conditioning in naive rats, proestrous (high estrogen) females froze significantly less compared with metestrous (low estrogen) females (Fig. 1D, main effect time, F(10,220) = 38.09, p < 0.0001; main effect of estrous cycle stage, F(1,22) = 7.127, *p = 0.014, and main interaction, F(10,220) = 1.947, *p = 0.0404). Similarly, during extinction, proestrous females also had lower freezing overall (Fig. 1E, main effect of time, F(10,220) = 11.44, p < 0.0001; and main interaction, F(10,220) = 2.573, p = 0.0058). To test whether these differences across estrous rely on BLA-BNST, this pathway was inhibited before training or extinction. When BLA-BNST neurons were inhibited during extinction, there was a main effect of estrous cycle stage, but not CNO treatment, as proestrous females froze significantly less than metestrous females during the first half of the tone (gray box 1; Fig. 8; main effect of estrous cycle stage, F(1,18) = 6.143, p = 0.0233). When BLA-BNST neurons were inhibited during prolonged cued fear training, there was no change in proestrus females, but there was reduced freezing behavior in metestrous females, although there was similar peak freezing by the end of training and these rats displayed similar freezing during subsequent extinction testing (Fig. 9). This might hint to a contribution of BLA-BNST to freezing in metestrous females on exposure to footshock, but this apparently did not translate into a change in learning. OF exploration was not affected by CNO treatment in either estrous cycle stage (Fig. 6). Similarly, SI behavior was not significantly affected by BLA-BNST inhibition in either estrous cycle stage (Fig. 7). Together, these data suggest that there may be a small effect of BLA-BNST inhibition limited to metestrous, but that apparent lack of effect on various behaviors in females is not the result of combining estrous cycle stages.
Figure 8.
Inhibition of BLA-BNST neurons before testing does not alter freezing in female rats. A, Rats were conditioned as described previously (see Fig. 1A), and CNO was administered before testing. Proestrous females spent significantly less time freezing compared with metestrous females during the training session (data not shown; main effect of time, F(4.748,94.97) = 38.83, p < 0.0001; main effect of estrous cycle stage, F(1,20) = 12.47, p = 0.0021; main interaction, F(10,200) = 2.315, p = 0.0135, two-way RM ANOVA). When females were separated by estrous cycle stage, there was only a main effect of time for proestrous (B; main interaction, F(10,100) = 0.3377, p = 0.9687; main effect of time, F(3.774,37.74) = 14.74, p < 0.0001; main effect of drug, F(1,10) = 1.12, p = 0.3148, two-way RM ANOVA), and metestrous (D; main interaction, F(10,80) = 0.8596, p = 0.5738; main effect of time, F(2.638,21.1) = 20.69, p < 0.0001; main effect of drug, F(1,8) = 0.9287, p = 0.3634, two-way RM ANOVA) during the training session. During testing, there was also only a main effect of time in proestrous (C; main interaction, F(10,100) = 0.7198, p = 0.7041; main effect of time, F(2.419,24.19) = 4.056, p = 0.0242; main effect of drug, F(1,10) = 1.346, p = 0.273, two-way RM ANOVA) and metestrous (E; main interaction, F(10,80) = 0.5362, p = 0.8595; main effect of time, F(22.542,20.33) = 6.842, p = 0.0033; main effect of drug, F(1,8) = 2.237, p = 0.1731, two-way RM ANOVA) rats. The total time spent freezing during baseline (F; main interaction, F(1,18) = 1.332, p = 0.2635; main effect of cycle stage, F(1,18) = 0.4348, p = 0.518; main effect of drug, F(1,18) = 0.07242, p = 0.7909, two-way ANOVA), the first half of the tone (G; main interaction, F(1,18) = 2.48, p = 0.1327; main effect of cycle, F(1,18) = 6.143, *p = 0.0233; main effect of drug, F(1,18) = 1.009, p = 0.3285, two-way ANOVA), second half of the tone (H; main interaction, F(1,18) = 2.122, p = 0.1624; main effect of cycle, F(1,18) = 0.0789, p = 0.782; main effect of drug, F(1,18) = 0.1433, p = 0.7095, two-way ANOVA), and post-test (I; main interaction, F(1,18) = 3.83, p = 0.066; main effect of cycle, F(1,18) = 1.558, p = 0.228; main effect of drug, F(1,18) = 0.08564, p = 0.7731, two-way ANOVA) were also analyzed separately. During the first half of the tone, metestrous females froze significantly more that proestrous females regardless of BLA-BNST inhibition.
Figure 9.
Inhibition of BLA-BNST neurons during prolonged cued fear training reduces freezing in metestrous females. A, Proestrous (N = 9) and metestrous (N = 19) female rats were injected with either saline (black) or CNO (gray) before training as described previously (Fig. 1A). BLA-BNST inactivation had no effect on freezing behavior in proestrous females (B; main interaction, F(10,70) = 0.5992, p = 0.8093; main effect of time, F(3.120,21.84) = 11.29, p < 0.0001; main effect of drug, F(1,7) = 0.1793, p = 0.6847, two-way RM ANOVA) and testing sessions (C; main interaction, F(10,70) = 0.7499, p = 0.6754; main effect of time, F(2.409,16.86) = 3.528, p = 0.0455; main effect of drug, F(1,7) = 0.8542, p = 0.3861, two-way RM ANOVA). D, However, in metestrous females, BLA-BNST inhibition significantly reduced freezing during training (main interaction, F(10,170) = 1.817, p = 0.0609; main effect of time, F(4.085,69.44) = 4.41 = 18.31, p < 0.0001; main effect of drug, F(1,17) = 6.172, *p = 0.0237, two-way RM ANOVA). E, Interestingly, this significant impact on training did not affect metestrous female freezing behavior during testing (main interaction, F(10,170) = 0.6373, p = 0.7804; main effect of time, F(23.547,60.3) = 8.395, p < 0.0001; main effect of drug, F(1,17) = 1.170, p = 0.2944, two-way RM ANOVA).
These data demonstrate that BLA-BNST neurons contribute to sustained fear responses as well as SI behavior, but differently in males and females. This might indicate that the activity of this pathway is different in females compared with males. Therefore, we sought to determine whether there were sex differences in the activity of BLA neurons that project to the BNST.
BLA-BNST neurons are less active and excitable in females compared with males
Previous studies in our laboratory have demonstrated that BLA neurons are more active in females compared with males (Blume et al., 2017). We used in vivo single-unit electrophysiology to measure neuronal activity of BLA neurons that project to the BNST. Antidromic stimulation was used to identify BLA neurons projecting to the BNST in naive male and female rats (Fig. 10A). We found that, while spontaneously firing BLA neurons were more active in females compared with males, BNST-projecting neurons were less active in females compared with males (Fig. 10B-E). While the majority of identified BLA-BNST neurons were located in the basal nucleus of the BLA (BA; Fig. 10F,G), a few neurons projecting to the BNST were also located in the lateral amygdala (LA). The activity of both BA- and LA-BNST neurons was consistently lower in females compared with males (Fig. 10B), and there were also significantly fewer identified BLA-BNST neurons per electrode penetration track in females compared with males (Fig. 10C; t(16) = 4.206, **p = 0.0006). In addition, the activity of identified BLA-BNST neurons in males was significantly higher than other BLA neurons that did not project to BNST (adjacent neurons; Fig. 10E; main interaction between sex and neuron type, F(1,132) = 4.357, p = 0.041). These data suggest there is a sex difference in the activity of BLA-BNST neurons, with these neurons being more active in males, but what exactly contributes to this difference is unknown.
Figure 10.
BNST-projecting BLA neurons are less active in females compared with males. Single-unit in vivo electrophysiological recordings were performed in male (N = 13) and female (N = 11) rats. A, Representative trace recording of an antidromically stimulated BLA-BNST neuron in which BNST stimulation (0.9 mA) successively elicits a corresponding BLA AP. Antidromic responses were identified as having 100% firing response probability and a <1 ms variability in latency during repeated stimulation (0.1 Hz). B, Spontaneously firing BLA-BNST neurons fired at a higher frequency in males compared with females (t(33.97) = 1.859, p = 0.0717, unpaired t test with Welch's correction). C, The average number of BLA-BNST neurons per track was significantly higher in males compared with females (t(16) = 4.206, **p < 0.001, unpaired t test with Welch's correction). E, There was a significant interaction between sex and neuron type when comparing BNST-projecting neurons with other BLA neurons as this specific subpopulation of neurons fired at a higher frequency compared with other BLA neurons in males, but at a lower frequency in females (main interaction, F(1,132) = 4.357, *p = 0.041; main effect of sex, F(1,132) = 0.3048, p = 0.5818; main effect of neuron type, F(1,132) = 0.2541, p = 0.6152, two-way ANOVA). D, This is also represented by the individual trace recordings from spontaneously active BLA neurons in male (top left) and female (top right) rats as well as an example BLA-BNST neuron from a male (bottom left) and female (bottom right) rat. F, Location of BNST stimulation probe during recordings for males (N = 26, blue) and females (N = 11, red) and example of histologic verification of stimulation probe within the BNST (right). G, Location for BNST-projecting BLA neurons for males (N = 26, blue) and females (N = 11, red) and example of histologic verification of Pontamine Sky Blue dye ejected in the BLA recording site at 10× (right). Numbers indicate the bregma coordinates from the atlas of Paxinos and Watson (2007).
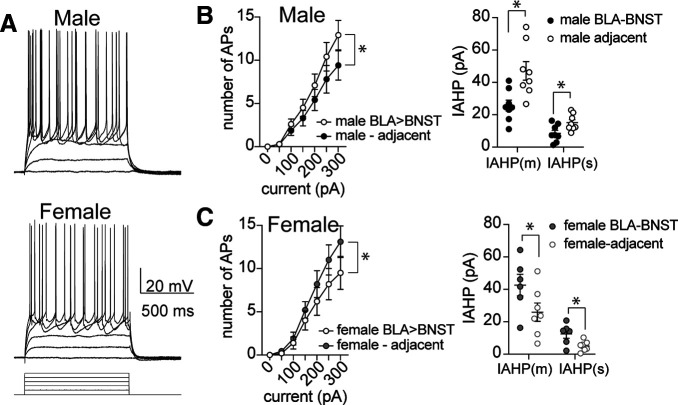
Therefore, the next set of experiments used ex vivo whole-cell patch-clamp recordings to measure excitability of BLA neurons. We found that, with increasing current, more APs were generated in BLA neurons from females compared with males (Fig. 11A–C; main effect of sex, F(1,217) = 14.18, p = 0.0002). This demonstrates that BLA neurons in females are more excitable than males, and is comparable to what we observed in the activity of unidentified neurons in single-unit in vivo recordings (Fig. 11E). Furthermore, when we examined the AHP potentials, females also had smaller AHP amplitudes (Fig. 11D; main effect of sex, F(1,42) = 9.65, p = 0.0034). When the AHP current (IAHP) was evoked by a brief voltage step in BLA neurons, females also had significantly lower amplitude of the medium and slow AHP currents (IAHP(m) and IAHP(s)) compared with males (Fig. 11E; main effect of sex, F(1,29) = 11.32, p = 0.0022), which may contribute to the observed differences in neuronal activity between males and females within the BLA. In a subset of rats, retrograde tracer beads were injected into the BNST 10 d before ex vivo electrophysiology experiments. The retrobeads enabled visual identification of BNST-projecting BLA neurons to allow assessment of neuronal excitability within this specific subpopulation. BLA-BNST neurons from males were more excitable than adjacent non-BLA-BNST neurons (Fig. 12A,B; main effect of neuronal type, F(1,140) = 5.749, p = 0.018). Moreover, this greater excitability coincided with smaller IAHPs in male BLA-BNST neurons compared with other BLA neurons (F(1,28) = 15.44, p = 0.001). The opposite pattern was seen in females. BLA-BNST neurons in females were less excitable than adjacent BLA neurons (Fig. 12C; F(1,98) = 5.0, p = 0.028) and showed larger IAHP amplitudes (F(1,22) = 7.0, p = 0.015). Together, these data support our behavioral findings in which BLA-BNST neuronal suppression reduced conditioned freezing during sustained fear extinction and promoted SI behavior in males, but not females. These data suggest that the sex differences in the excitability of BLA-BNST between males and females could underlie sex differences in the patterns of behavioral responses.
Figure 11.
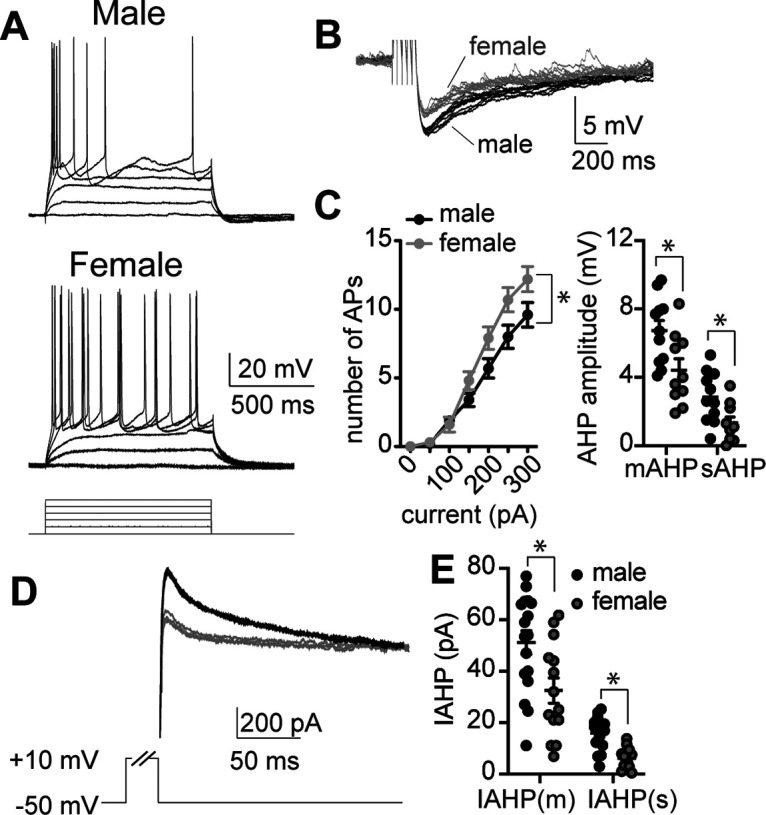
BLA neurons are more excitable and have smaller AHPs in females. A, Representative traces of BLA neuron firing responses in response to a range of depolarizing current steps in BLA neurons from male (top) and female rats (bottom). B, Representative traces of AHPs recorded from BLA neurons of male (black) and female rats (gray) following a train of 5 APs. C, BLA neurons recorded from females were more excitable than males over a range of current steps (left; main effect of sex, p = 0.0002, F(1,217) = 14.18, N = 17 neurons males, N = 16 neurons females, two-way ANOVA), and this coincided with BLA neurons from females having a smaller medium and slow afterhyperpolarization amplitude (mAHP and sAHP, respectively; main effect of sex, p = 0.0034, F(1,42) = 9.65, N = 12 neurons males, N = 11 neurons females, two-way ANOVA). D, Representative traces of the AHP current (IAHP) measured in BLA neurons from male (black) and female rats (gray) evoked by a brief voltage step. E, The amplitude of the medium and slow IAHP currents (IAHP(m) and IAHP(s)) is significantly lower in BLA neurons recorded from female rats compared with males (main effect of sex, p = 0.0022, F(1,29) = 11.32, N = 17 neurons males, N = 14 neurons females, two-way ANOVA).
Figure 12.
BLA-BNST neurons are more excitable and have smaller AHPs in males. A, Representative traces of BLA-BNST neuron firing in response to a range of depolarizing current steps recorded from male (top) and female rats (bottom). B, BLA-BNST neurons recorded from males (N = 11 neurons, white) are more excitable than adjacent unlabeled BLA neurons (N = 11 neurons, black; left; p = 0.018, F(1,140) = 5.749, two-way ANOVA) and have smaller IAHP amplitude (right; p = 0.001, F(1,28) = 15.44, N = 8 neurons/group, two-way ANOVA). C, BLA-BNST neurons recorded from females (N = 8, white) are less excitable (left) compared with adjacent BLA neurons (N = 8, black; p = 0.028, F(1,98) = 5.0, two-way ANOVA) and show larger IAHP amplitude (right, p = 0.015, F(1,22) = 7.0, N = 6 or 7 neurons/group, two-way ANOVA).
Discussion
Phasic fear can shift into a state of sustained apprehension, and the unpredictability associated with diffuse cues within the environment can contribute to sustained anxiety. The neurocircuitry underlying the shift from phasic to sustained anxiety is not well defined, although recently the BNST is implicated as being important in the shift to a sustained anxiety state (Walker et al., 2009; Davis et al., 2010; Alvarez et al., 2011; Goode et al., 2019). Here we demonstrate that BLA-BNST circuitry is necessary for the expression of sustained anxiety-like behavior. Reversible inhibition of BLA-BNST neurons during prolonged cued fear testing suppressed conditioned freezing behavior in males, whereas it did not reduce general anxiety-like behavior in the OF compared with controls. However, SI was increased in males. Interestingly, BLA-BNST inhibition had little effect on any of the behaviors measured in females (and might even have increased conditioning freezing), which suggests that BLA-BNST neurons have an attenuated role during sustained anxiety in females. This is further supported by the finding that BLA-BNST neurons in males are more excitable and have smaller IAHPs than those from females where BLA-BNST neurons are less active and excitable compared with general BLA neuron population. Together, these results demonstrate sex differences in the activity of BLA-BNST neurons, which provide a cellular basis for understanding a potential role for the BLA-BNST in the generation of sustained anxiety-like behavior in males, but not females.
Earlier reports demonstrate that both temporary and permanent inactivation of the BNST does not affect the acquisition of cued or contextual fear (LeDoux et al., 1988; Duvarci et al., 2009; Pelrine et al., 2016). However, the BNST contributes to the expression of fear memories, and the BLA-BNST pathway is necessary for contextual fear (Sullivan et al., 2004; Haufler et al., 2013; Sasaki Russell et al., 2020). Our results further support a key role for BLA-BNST projections in the expression of freezing during a sustained conditioned cue, independent of the context, but only in males. To our knowledge, this is the first characterization of sex differences in specific neurocircuits involved in anticipatory anxiety. The observed sex differences, with females freezing less during testing of the response to a prolonged cue, is consistent with the parallel dampening effect of BLA-BNST inactivation in males and the greater responses to prolonged anxiogenic cues in males.
The BNST exhibits sex-dependent molecular and behavioral responses to stress (Allen and Gorski, 1990; Bangasser and Shors, 2008; Duque-Wilckens et al., 2018; Mavrikaki et al., 2019; Luo et al., 2021). Therefore, the minimal effect of BLA-BNST inhibition on freezing behavior in females was not completely unexpected. Waddell et al. (2006) found that BNST lesions in female rats impaired extinction to long, but not short, duration cues. Although this study did not assess the contribution of BLA-BNST projections, it supported a role for BNST in sustained anxiety in females, but perhaps not through direct BLA input. In previous studies, darting better reflects fear expression in females (Gruene et al., 2015); however, darting behavior was not altered in these or our previous studies (Blume et al., 2017). Importantly, females and males display similar levels of freezing throughout conditioning and in the onset of the CS during recall. However, these responses rapidly diverge in males in the apparent rate of acquisition of extinction to the prolonged cue. So, sex differences in the effect of BLA-BNST inhibition are not because of an inability to freeze. Furthermore, during extinction, metestrous females showed freezing levels that were equal to males but were not altered by BLA-BNST inhibition. This further supports a clear difference in the role of BLA-BNST in the sexes and hints toward a hormonal influence on this behavior.
Anticipation of a social situation can manifest as social anxiety. In human and nonhuman primates, the BNST is consistently more active in individuals displaying social anxiety (J. Clauss, 2019; J.A. Clauss et al., 2019). Our results are congruent with these findings and further suggest that BLA input into the BNST contributes to social anxiety in males. Here, male rats, in which BLA-BNST neurons were inhibited during SI, had a reduced latency to interact and spent more time interacting compared with controls. However, these responses were not observed in female rats, as their SI behavior was not affected by treatment nor estrous cycle stage. It is plausible that BLA-BNST inhibition was not able to reduce the latency to interact in females because their latencies were already low compared with males. However, both control females and males spend similar amounts of time interacting, yet BLA-BNST inhibition only increases time spent interacting in males. These results are consistent with nonhuman primate and rodent studies in which inactivation of the amygdala increases SI (Woolley et al., 2006; Felix-Ortiz et al., 2016; Paine et al., 2017; Elorette et al., 2020; Folkes et al., 2020) and activation of the amygdala attenuates SI (Felix-Ortiz and Tye, 2014; Felix-Ortiz et al., 2016; Paine et al., 2017). Our current results also expand on prior studies in which chemogenetic and pharmacological activation of the BNST reduces SI in males (Lee et al., 2008; Lungwitz et al., 2012; Emmons et al., 2021). However, these are the first results, that we are aware of, that directly examine BLA-BNST neuronal inhibition on SI in both sexes and demonstrate a relatively weak role of BLA-BNST activity in stimulus-evoked anxiety, either from a cued or social source, in females.
A strong correlation between social dysfunction and anxiety disorders is supported by studies in humans and animals (Allsop et al., 2014). Therefore, it is reasonable to expect that inhibition of BLA-BNST neurons would decrease anxiety as reflected through increased center exploration of the OF in male rats. However, OF behaviors were not altered by BLA-BNST inhibition in either sex. While decreased BLA activity is consistently associated with reduced anxiety in the OF (Felix-Ortiz et al., 2016; Ranjbar et al., 2017; Yin et al., 2019), inhibition of the BNST has more variable effects on OF behavior. Pharmacological manipulations of BNST function impact SI, but not OF or elevated plus maze exploration (Lee et al., 2008; Crestani et al., 2010). This, together with our data, suggests that BLA-BNST projections are uniquely recruited during sustained anxiety-like states that occur during a social encounter or anticipated threats.
The BLA exhibits a dynamic range of neuronal activity in response to environmental stimuli, such as conditioned cues, to promote freezing (Maren, 2001; Pape and Pare, 2010; Sun et al., 2020). Consequently, dysregulation of BLA outputs may drive maladaptive behaviors, such as sustained anxiety. It is well established in humans and rodent models that the amygdala and BNST are hyperactive in anxiety-like disorders (Rauch et al., 2003; Phan et al., 2006; Stein et al., 2007; Fox et al., 2018) and sex differences exist in the neuronal activity of these brain regions (Blume et al., 2017, 2019; Smithers et al., 2019). Prior electrophysiological experiments have established sex differences within the BLA and the BNST, but until now, limited data were available regarding the activity of specific BLA-BNST projections. Analogous to prior studies, we observed that BLA neurons from females had a higher in vivo firing frequency and were more excitable ex vivo compared with males (Blume et al., 2017, 2019; Ornelas and Keele, 2018). In contrast, a key finding of these results is that the in vivo firing rate of BLA-BNST neurons in females was lower than other BLA neurons, whereas BLA-BNST neurons from males had a higher firing frequency compared with other, non–BNST-projecting, BLA neurons. Consistent with these findings, BLA-BNST neurons in males were more excitable and had smaller IAHPs compared with other BLA neurons. These electrophysiological findings support our behavioral data where there is a larger impact of inactivation of the BLA-BNST projection in males with negligible effects noted on behavior in females. Notably, the excitability of BLA and LAT neurons is sensitive to fear conditioning (Rosenkranz and Grace, 2002; Motanis et al., 2014; Sehgal et al., 2014), so baseline excitability may impose constraints on the possible degree of plasticity of this property during fear conditioning. In addition, the already low BLA-BNST neuron activity in females may limit the behavioral impact of chemogenetic inhibition, and therefore can be reasoned that hyperactivation of the BLA-BNST in females, such as during stress, may have a greater impact on behaviors. Based on this interpretation and the evidence that chemogenetic inhibition impacted the excitability of BLA-BNST neurons equally in males and females, we favor the interpretation that chemogenetic suppression of BLA-BNST had small impact in female behavior because this pathway is not heavily recruited in these conditions in female rats. However, it is also possible that BLA-BNST in females is actively inhibited during these behaviors, so chemogenetic suppression was without effect.
The effects of stress on BNST-mediated behaviors in females is a key area of focus (Allen and Gorski, 1990; Bangasser and Shors, 2008; Duque-Wilckens et al., 2018; Mavrikaki et al., 2019; Luo et al., 2021); and while acute and chronic stressors alter BLA activity in a sex-dependent manner (Ornelas and Keele, 2018; Blume et al., 2019; Gupta and Chattarji, 2021), the specific contribution of BLA-BNST neurons to stress-related behavior and responses remains to be explored. This neurocircuit may be uniquely activated in females following stress to facilitate anxiety-like behaviors. The sexual divergence of this neurocircuit may account for the sex differences observed in anxiety disorders and requires further exploration to illuminate the neurobiology of anticipatory anxiety in females.
Footnotes
This work was supported by National Institutes of Health R01MH118237 to J.A.R. and R01MH100536 to J.A.R. and J.H.U. We thank Dr. Nicole Ferrara for technical assistance with viral injections and thoughtful discussions contributing to the interpretation of the data.
The authors declare no competing financial interests.
References
- Allen LS, Gorski RA (1990) Sex difference in the bed nucleus of the stria terminalis of the human brain. J Comp Neurol 302:697–706. [DOI] [PubMed] [Google Scholar]
- Allsop SA, Vander Weele CM, Wichmann R, Tye KM (2014) Optogenetic insights on the relationship between anxiety-related behaviors and social deficits. Front Behav Neurosci 8:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez RP, Chen G, Bodurka J, Kaplan R, Grillon C (2011) Phasic and sustained fear in humans elicits distinct patterns of brain activity. NeuroImage 55:389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasthi S, Pan H, LeDoux JE, Cloitre M, Altemus M, McEwen B, Silbersweig D, Stern E (2020) The bed nucleus of the stria terminalis and functionally linked neurocircuitry modulate emotion processing and HPA axis dysfunction in posttraumatic stress disorder. Neuroimage Clin 28:102442. 10.1016/j.nicl.2020.102442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandelow B, Michaelis S (2015) Epidemiology of anxiety disorders in the 21st century. Dialogues Clin Neurosci 17:327–335. 10.31887/DCNS.2015.17.3/bbandelow [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Shors TJ (2008) The bed nucleus of the stria terminalis modulates learning after stress in masculinized but not cycling females. J Neurosci 28:6383–6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume SR, Freedberg M, Vantrease JE, Chan R, Padival M, Record MJ, De Joseph MR, Urban JH, Rosenkranz JA (2017) Sex- And estrus-dependent differences in rat basolateral amygdala. J Neurosci 37:10567–10586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume SR, Padival M, Urban JH, Rosenkranz JA (2019) Disruptive effects of repeated stress on basolateral amygdala neurons and fear behavior across the estrous cycle in rats. Sci Rep 9:12292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann L, Buff C, Feldker K, Tupak SV, Becker MP, Herrmann MJ, Straube T (2017) Distinct phasic and sustained brain responses and connectivity of amygdala and bed nucleus of the stria terminalis during threat anticipation in panic disorder. Psychol Med 47:2675–2688. 10.1017/S0033291717001192 [DOI] [PubMed] [Google Scholar]
- Brunello N, Davidson JR, Deahl M, Kessler RC, Mendlewicz J, Racagni G, Shalev AY, Zohar J (2001) Posttraumatic stress disorder: diagnosis and epidemiology, comorbidity and social consequences, biology and treatment. Neuropsychobiology 43:150–162. 10.1159/000054884 [DOI] [PubMed] [Google Scholar]
- Clauss J (2019) Extending the neurocircuitry of behavioural inhibition: a role for the bed nucleus of the stria terminalis in risk for anxiety disorders. Gen Psychiatry 32:e100137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss JA, Avery SN, Benningfield MM, Blackford JU (2019) Social anxiety is associated with BNST response to unpredictability. Depress Anxiety 36:666–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani CC, Alves FHF, Correa FMA, Guimarães FS, Joca SRL (2010) Acute reversible inactivation of the bed nucleus of stria terminalis induces antidepressant-like effect in the rat forced swimming test. Behav Brain Funct 6:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daldrup T, Lesting J, Meuth P, Seidenbecher T, Pape HC (2016) Neuronal correlates of sustained fear in the anterolateral part of the bed nucleus of stria terminalis. Neurobiol Learn Mem 131:137–146. 10.1016/j.nlm.2016.03.020 [DOI] [PubMed] [Google Scholar]
- Dalla C, Shors TJ (2009) Sex differences in learning processes of classical and operant conditioning. Physiol Behav 97:229–238. 10.1016/j.physbeh.2009.02.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C (2010) Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology 35:105–135. 10.1038/npp.2009.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvarci S, Bauer EP, Paré D (2009) The bed nucleus of the stria terminalis mediates inter-individual variations in anxiety and fear. Neurosci 29:10357–10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque-Wilckens N, Steinman MQ, Busnelli M, Chini B, Yokoyama S, Pham M, Laredo SA, Hao R, Perkeybile AM, Minie VA, Tan PB, Bales KL, Trainor BC (2018) Oxytocin receptors in the anteromedial bed nucleus of the stria terminalis promote stress-induced social avoidance in female California mice. Biol Psychiatry 83:203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elorette C, Aguilar BL, Novak V, Forcelli PA, Malkova L (2020) Dysregulation of behavioral and autonomic responses to emotional and social stimuli following bidirectional pharmacological manipulation of the basolateral amygdala in macaques. Neuropharmacology 179:108275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmons R, Sadok T, Rovero NG, Belnap MA, Henderson HJM, Quan AJ, Del Toro NJ, Halladay LR (2021) Chemogenetic manipulation of the bed nucleus of the stria terminalis counteracts social behavioral deficits induced by early life stress in C57BL/6J mice. J Neurosci Res 99:90–109. [DOI] [PubMed] [Google Scholar]
- Fani N, Tone EB, Phifer J, Norrholm SD, Bradley B, Ressler KJ, Kamkwalala A, Jovanovic T (2012) Attention bias toward threat is associated with exaggerated fear expression and impaired extinction in PTSD. Psychol Med 42:533–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix-Ortiz AC, Burgos-Robles A, Bhagat ND, Leppla CA, Tye KM (2016) Bidirectional modulation of anxiety-related and social behaviors by amygdala projections to the medial prefrontal cortex. Neuroscience 321:197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix-Ortiz Ada C, Tye KM (2014) Amygdala Inputs to the Ventral Hippocampus Bidirectionally Modulate Social Behavior. J Neurosci 34:586–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenster RJ, Lebois LA, Ressler KJ, Suh J (2018) Brain circuit dysfunction in post-traumatic stress disorder: from mouse to man. Nat Rev Neurosci 19:535–551. 10.1038/s41583-018-0039-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkes OM, Báldi R, Kondev V, Marcus DJ, Hartley ND, Turner BD, Ayers JK, Baechle JJ, Misra MP, Altemus M, Grueter CA, Grueter BA, Patel S (2020) An endocannabinoid-regulated basolateral amygdala-nucleus accumbens circuit modulates sociability. J Clin Investig 130:1728–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, Oler JA, Birn RM, Shackman AJ, Alexander AL, Kalin NH (2018) Functional connectivity within the primate Extended Amygdala Is Heritable and associated with early-life anxious temperament. J Neurosci 38:7611–7621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode TD, Ressler RL, Acca GM, Miles OW, Maren S (2019) Bed nucleus of the stria terminalis regulates fear to unpredictable threat signals. Elife 8:e46525. 10.7554/eLife.46525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruene TM, Flick K, Stefano A, Shea SD, Shansky RM (2015) Sexually divergent expression of active and passive conditioned fear responses in rats. Elife 4:e11352. 10.7554/eLife.11352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta K, Chattarji S (2021) Sex differences in the delayed impact of acute stress on the amygdala. Neurobiol Stress 14:100292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haufler D, Nagy FZ, Pare D (2013) Neuronal correlates of fear conditioning in the bed nucleus of the stria terminalis. Learn Mem 20:633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann MJ, Boehme S, Becker MP, Tupak SV, Guhn A, Schmidt B, Brinkmann L, Straube T (2016) Phasic and sustained brain responses in the amygdala and the bed nucleus of the stria terminalis during threat anticipation. Hum Brain Mapp 37:1091–1102. 10.1002/hbm.23088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, Bach DR, Esposito F, Di Salle F, Perrig WJ, Scheffler K, Lüthi A, Seifritz E (2007) Processing of temporal unpredictability in human and animal amygdala. J Neurosci 27:5958–5966. 10.1523/JNEUROSCI.5218-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetzel A, Meredith GE, Rademacher DJ, Rosenkranz JA (2012) Effect of amphetamine place conditioning on excitatory synaptic events in the basolateral amygdala ex vivo. Neuroscience 206:7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston AL, File SE (1991) Sex differences in animal tests of anxiety. Physiol Behav 49:245–250. 10.1016/0031-9384(91)90039-Q [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Ressler KJ (2010) How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. Am J Psychiatry 167:648–662. 10.1176/appi.ajp.2009.09071074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE (2005) Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62:593–602. 10.1001/archpsyc.62.6.593 [DOI] [PubMed] [Google Scholar]
- Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN, Whalen PJ (2011) The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behav Brain Res 223:403–410. 10.1016/j.bbr.2011.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Adhikari A, Lee SY, Marshel JH, Kim CK, Mallory CS, Lo M, Pak S, Mattis J, Lim BK, Malenka RC, Warden MR, Neve R, Tye KM, Deisseroth K (2013) Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature 496:219–223. 10.1038/nature12018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange MD, Daldrup T, Remmers F, Szkudlarek HJ, Lesting J, Guggenhuber S, Ruehle S, Jüngling K, Seidenbecher T, Lutz B, Pape HC (2017) Cannabinoid CB1 receptors in distinct circuits of the extended amygdala determine fear responsiveness to unpredictable threat. Mol Psychiatry 22:1422–1430. 10.1038/mp.2016.156 [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Iwata J, Cicchetti P, Reis DJ (1988) Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci 8:2517–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Fitz S, Johnson PL, Shekhar A (2008) Repeated stimulation of CRF receptors in the BNST of rats selectively induces social but not panic-like anxiety. Neuropsychopharmacology 33:2586–2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovick TA, Zangrossi H (2021) Effect of estrous cycle on behavior of females in rodent tests of anxiety. Front Psychiatry 12:711065. 10.3389/fpsyt.2021.711065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lungwitz EA, Molosh A, Johnson PL, Harvey BP, Dirks RC, Dietrich A, Minick P, Shekhar A, Truitt WA (2012) Orexin-A induces anxiety-like behavior through interactions with glutamatergic receptors in the bed nucleus of the stria terminalis of rats. Physiol Behav 107:726–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo PX, Manning CE, Fass JN, Williams AV, Hao R, Campi KL, Trainor BC (2021) Sex-specific effects of social defeat stress on miRNA expression in the anterior BNST. Behav Brain Res 401:113084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavrikaki M, Pantano L, Potter D, Rogers-Grazado MA, Anastasiadou E, Slack FJ, Amr SS, Ressler KJ, Daskalakis NP, Chartoff E (2019) Sex-Dependent changes in miRNA expression in the bed nucleus of the stria terminalis following stress. Front Behav Neurosci 12:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ (1982) Neurons of the lateral and basolateral amygdaloid nuclei: a Golgi study in the rat. J Comp Neurol 212:293–312. [DOI] [PubMed] [Google Scholar]
- McDonald AJ (1984) Neuronal organization of the lateral and basolateral amygdaloid nuclei in the rat. J Comp Neurol 222:589–606. [DOI] [PubMed] [Google Scholar]
- Michely J, Rigoli F, Rutledge RB, Hauser TU, Dolan RJ (2020) Distinct processing of aversive experience in amygdala subregions. Biol Psychiatry Cogn Neurosci Neuroimaging 5:291–300. 10.1016/j.bpsc.2019.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S (2001) Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci 24:897–931. [DOI] [PubMed] [Google Scholar]
- Motanis H, Maroun M, Barkai E (2014) Learning-induced bidirectional plasticity of intrinsic neuronal excitability reflects the valence of the outcome. Cereb Cortex 24:1075–1087. 10.1093/cercor/bhs394 [DOI] [PubMed] [Google Scholar]
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals (2011) Guide for the care and use of laboratory animals, Ed 8. Washington (DC): National Academies Press (US). [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T (2018) Fear processing, psychophysiology, and PTSD. Harv Rev Psychiatry 26:129–141. 10.1097/HRP.0000000000000189 [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Glover EM, Stevens JS, Fani N, Galatzer-Levy IR, Bradley B, Ressler KJ, Jovanovic T (2015) Fear load: the psychophysiological over-expression of fear as an intermediate phenotype associated with trauma reactions. Int J Psychophysiol 98:270–275. 10.1016/j.ijpsycho.2014.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornelas LC, Keele NB (2018) Sex differences in the physiological response to ethanol of rat basolateral amygdala neurons following single-prolonged stress. Front Cell Neurosci 12:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine TA, Swedlow N, Swetschinski L (2017) Decreasing GABA function within the medial prefrontal cortex or basolateral amygdala decreases sociability. Behav Brain Res 317:542–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape H-C, Pare D (2010) Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev 90:419–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palanza P (2001) Animal models of anxiety and depression: how are females different? Neurosci Biobehav Rev 25:219–233. 10.1016/S0149-7634(01)00010-0 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C (2007) The rat brain in stereotaxic coordinates, Ed 6. San Diego: Elsevier Academic Press. [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Tancer ME (2006) Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biol Psychiatry 59:424–429. [DOI] [PubMed] [Google Scholar]
- Pelrine E, Pasik SD, Bayat L, Goldschmiedt D, Bauer EP (2016) 5-HT2C receptors in the BNST are necessary for the enhancement of fear learning by selective serotonin reuptake inhibitors. Neurobiology of Learning and Memory 136:189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perusini JN, Fanselow MS (2015) Neurobehavioral perspectives on the distinction between fear and anxiety. Learn Mem 22:417–425. 10.1101/lm.039180.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajbhandari AK, Gonzalez ST, Fanselow MS (2018) Stress-enhanced fear learning, a robust rodent model of post-traumatic stress disorder. J Vis Exp 140:58306. 10.3791/58306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjbar H, Radahmadi M, Reisi P, Alaei H (2017) Effects of electrical lesion of basolateral amygdala nucleus on rat anxiety-like behaviour under acute, sub-chronic, and chronic stresses. Clin Exp Pharmacol Physiol 44:470–479. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Wright CI (2003) Neuroimaging studies of amygdala function in anxiety disorders. Ann N Y Acad Sci 985:389–410. [DOI] [PubMed] [Google Scholar]
- Remes O, Brayne C, van der Linde R, Lafortune L (2016) A systematic review of reviews on the prevalence of anxiety disorders in adult populations. Brain Behav 6:e00497. 10.1002/brb3.497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA (2002) Dopamine-mediated modulation of odour-evoked amygdala potentials during pavlovian conditioning. Nature 417:282–287. 10.1038/417282a [DOI] [PubMed] [Google Scholar]
- Salvatore M, Wiersielis KR, Luz S, Waxler DE, Bhatnagar S, Bangasser DA (2018) Sex differences in circuits activated by corticotropin releasing factor in rats. Horm Behav 97:145–153. 10.1016/j.yhbeh.2017.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Russell J, Trouche S, Reijmers LG (2020) Functional characterization of the basal amygdala-dorsal BNST pathway during contextual fear conditioning. eNeuro 7:ENEURO.0163-20.2020. 10.1523/ENEURO.0163-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal M, Ehlers VL, Moyer JRJ (2014) Learning enhances intrinsic excitability in a subset of lateral amygdala neurons. Learn Mem 21:161–170. 10.1101/lm.032730.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithers HE, Terry JR, Brown JT, Randall AD (2019) Sex-associated differences in excitability within the bed nucleus of the stria terminalis are reflective of cell-type. Neurobiol Stress 10:100143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack A, Carrier N, Dietz D, Hollis F, Sorenson J, Kabbaj M (2010) Sex differences in social interaction in rats: role of the immediate-early gene zif268. Neuropsychopharmacology 35:570–580. 10.1038/npp.2009.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Simmons AN, Feinstein JS, Paulus MP (2007) Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry 164:318–327. [DOI] [PubMed] [Google Scholar]
- Sullivan GM, Apergis J, Bush DEA, Johnson LR, Hou M, Ledoux JE (2004) Lesions in the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience 128:7–14. [DOI] [PubMed] [Google Scholar]
- Sun Y, Gooch H, Sah P (2020) Fear conditioning and the basolateral amygdala. F1000Research 9:F1000 Faculty Rev-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrisi S, Gorka AX, Gonzalez-Castillo J, O'Connell K, Balderston N, Grillon C, Ernst M (2018) Extended amygdala connectivity changes during sustained shock anticipation. Transl Psychiatry 8:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell J, Morris RW, Bouton ME (2006) Effects of bed nucleus of the stria terminalis lesions on conditioned anxiety: aversive conditioning with long-duration conditional stimuli and reinstatement of extinguished fear. Behav Neurosci 120:324–336. 10.1037/0735-7044.120.2.324 [DOI] [PubMed] [Google Scholar]
- Walker DL, Miles LA, Davis M (2009) Selective participation of the bed nucleus of the stria terminalis and CRF in sustained anxiety-like versus phasic fear-like responses. Prog Neuropsychopharmacol Biol Psychiatry 33:1291–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley ML, Haman M, Higgins GA, Ballard TM (2006) Investigating the effect of bilateral amygdala lesions on fear conditioning and social interaction in the male Mongolian gerbil. Brain Research 1078:151–158. [DOI] [PubMed] [Google Scholar]
- Yin F, Guo H, Cui J, Shi Y, Su R, Xie Q, Chang J, Wang Y, Lai J (2019) The basolateral amygdala regulation of complex cognitive behaviours in the five-choice serial reaction time task. Psychopharmacology 236:3135–3146. [DOI] [PubMed] [Google Scholar]
- Zimmerberg B, Farley MJ (1993) Sex differences in anxiety behavior in rats: role of gonadal hormones. Physiol Behav 54:1119–1124. 10.1016/0031-9384(93)90335-D [DOI] [PubMed] [Google Scholar]
- Zimmerman JM, Maren S (2011) The bed nucleus of the stria terminalis is required for the expression of contextual but not auditory freezing in rats with basolateral amygdala lesions. Neurobiol Learn Mem 95:199–205. 10.1016/j.nlm.2010.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]