Extended Data Fig. 1. Multiple configurations of mWGA-based tracer were examined for anterograde synaptic transfer efficiency in the retinotectal synapses.
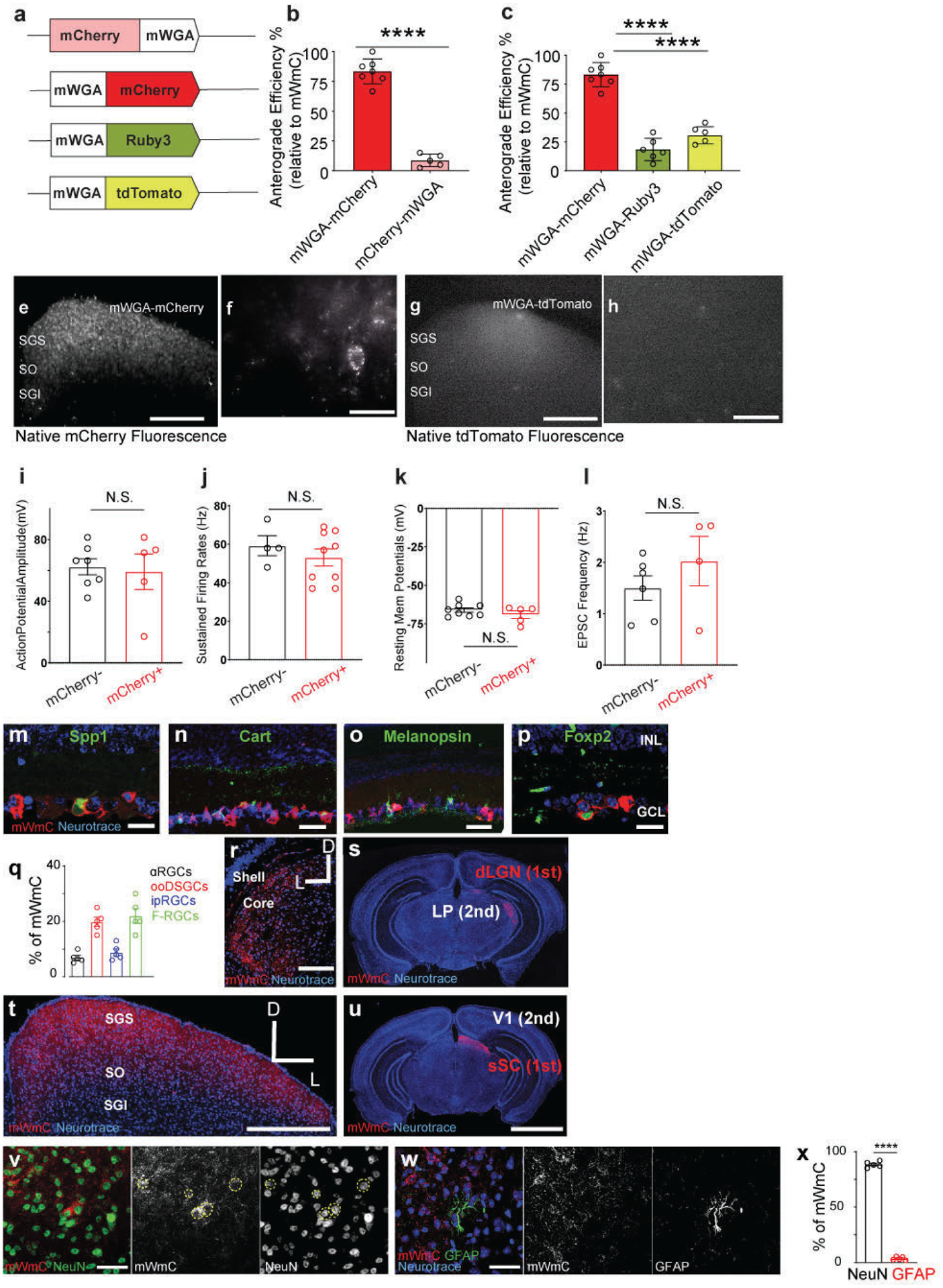
The numbers of fluorescence-positive neurons in the SGS from different mWGA-RFP configurations were counted. The percentages were normalized to the highest number as (100%) set by the mWGA-mCherry configuration, which possessed the highest efficiency. The anterograde transfer number was quantified by counting the number of RFP-positive neurons in acute SC slices on the contralateral side (right SC) without fluorescence amplification through immunostaining. We focused on the contralateral (right) SC, as 90% of RGCs project contralaterally31. We examined RGC coverage across the retina to ensure there was no retinotopy bias in SC analysis. mWGA-fusion protein configurations were compared under consistent conditions by delivering AAV2 expressing the fusion proteins into the left eyes (Fig. 1a). We used standard AAV Serotype-2 followed by 4 weeks post-injection (wpi) sampling, a commonly established window to examine RGC axonal projections and carry out the optogenetic measurement8. a, Several WGA-fusion proteins were compared side-by-side using an AAV-mediated in vivo screen for highly efficient anterograde transfer from the retina to the brain. b, Comparisons between N-terminal (mCherry-mWGA) and C-terminal fusion (mWGA-mCherry) transfer efficiencies were quantified. c, Transfer efficiencies of different RFP C-terminal fusions, including mWGA-mCherry, mWGA-Ruby3, mWGA-tdTmt, were quantified, n=4 animals for each condition. Statistics for b, two-sided Student’s t-test, ****, P<0.0001; c, one-way ANOVA test. ****, P<0.0001. e-f, (e) Additional samples showing live red-fluorescent labeling of the contralateral SC after acute brain slice preparation 4 weeks post-injection (wpi), indicating transsynaptic transfer onto the recipient neurons enriched in stratum griseum superficiale (SGS) and stratum opticum (SO), but not in stratum griseum intermedium (SGI). f, Magnified view of inset from e showing individual neurons labeled with bright red fluorescent protein from RGC anterograde transfer without signal amplification through immunostaining. The ability to detect native fluorescence is unique to mWGA-mCherry (mWmC) but absent in other mWGA-RFP configurations, such as mWGA-TdTomato shown here (g, h). Scale bars: (e, g, 5 mm; f, h, 50μm). i-l, Intrinsic electrophysiological properties of mCherry-positive recipient neurons (red, n=5 animals) and neighboring mCherry-negative neurons (black, n=4 animals) are similar as showed in Fig. 1m. i, Action potential amplitudes, j, sustained firing rates, k, resting membrane potentials, and l, EPSC frequencies are shown. These intrinsic properties were unperturbed by mWmC-transfer. N.S. not significant. two-sided Student’s t-test. m-p, Retinal vertical sections to show high mWmC coverage across major RGC types, including (m) Spp1 for αRGCs, (n) Cart for ooDSGCs, (o) Melanopsin for ipRGCs, and (p) Foxp2 for F-RGCs. Scale bar: 20 μm. INL: inner nuclear layer; GCL: ganglion cell layer. The percentages of each RGC subclasses were quantified in q, representing a similar fraction of RGC subclasses among all RGCs14, n=5 animals. f-i, Intraocular injections of mWmC lead to efficient monosynaptic transfer to connected neurons in multiple retino-recipient areas, including SC (t,u) and LGN (r, s). By contrast, the secondary relay neurons in V1 (u) or those in the lateral posterior nucleus of the thalamus (LP) (s) do not show mWmC transfer. Scale bars: (r, t, 2mm; s, u, 20mm). v, Immunostaining for RFP (mWmC) indicates high efficiency of anterograde transfer onto the recipient neurons in stratum griseum superficiale (SGS) and stratum opticum (SO). mWmC-positive cells are largely NeuN-positive. Dotted-yellow circles indicate mCherry and NeuN double-positive neurons from RGC anterograde transsynaptic transfer. w, mWmC-positive cells are largely GFAP-negative, which were quantified in x. n=5 animals for each condition. ****, p<0.0001, two-sided Student’s t-test. Scale bars: (v, w, 50μm). All data in this figure are presented as mean ± SEM.
