Figure 3.
In vivo evaluation of AdCAR-T
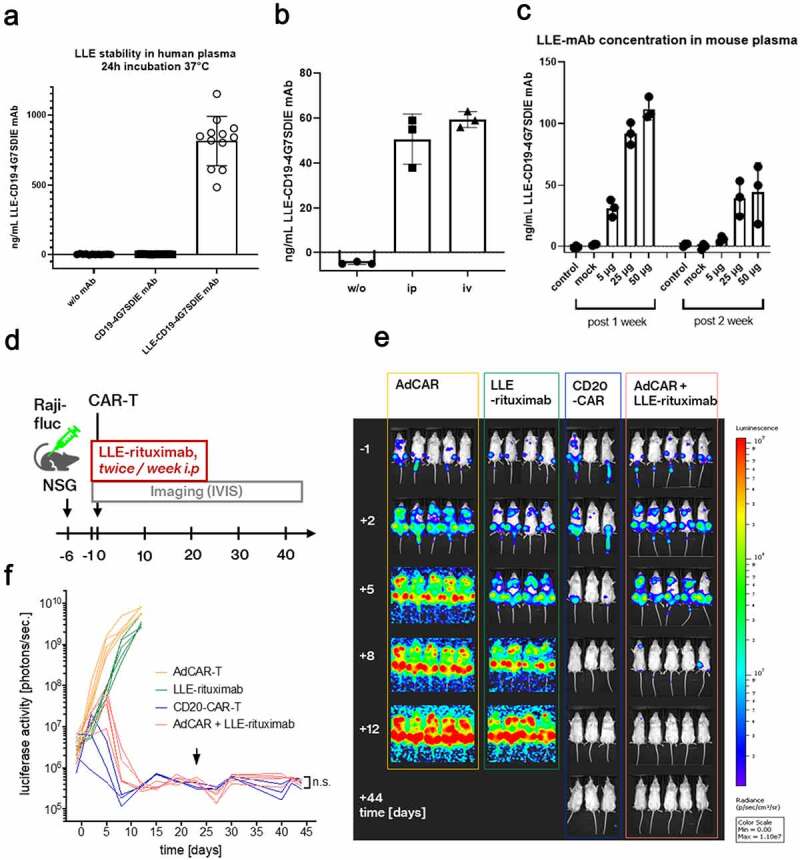
A) First we tested the stability and functionality of the LLE-conjugation to mAb in human whole blood at 37°C for 24 h (Figure 2a). The starting concentration was 1 µg/mL. We added 1 µg of LLE-CD19 mAb to freshly isolated human plasma (10 IU/mL sodium heparin) (n = 12) independent donors in one experiment. The LLE-mAb concentration was measured indirectly as the binding capacity to the cell line NALM6 with a secondary anti-biotin mAb calculated by a standard curve. The analysis revealed stability of the LLE-conjugation.B) Three mice received PBS (w/o LLE-mAb) or either 5 µg of LLE-CD19 mAb intraperitoneally or intravenously. After 24 h, the plasma levels of LLE-CD19 mAb were measured by flow cytometry. The comparison revealed no significant difference in the plasma levels. C) Three mice received PBS (w/o LLE-mAb) or CD19 mAb w/o LLE-conjugation or 5, 25, or 50 µg of LLE-CD19 mAb intraperitoneally. At the time points 7 days and 14 days, the plasma levels of LLE-CD19 mAb were measured by flow cytometry and were detectable after 14 days at relevant concentrations in a nontumor bearing NSG mouse model. D) Schematic illustration of the in vivo experiment: NSG mice were inoculated with the NHL cell line Raji (Raji-fluc)at day −6. At day −1, mice were randomized after in vivo imaging according to BLI activity (Supplementary Figure 3b). LLE-rituximab (50 µg) was injected intraperitoneally twice weekly as indicated, starting on day −1. Application was suspended on day +23. AdCAR-T or CD20-CAR-T were injected intravenously as indicated on day 0. E) Luciferase activity [photons/sec] was determined by in vivo BLI. Representative BL images of the study group, AdCAR-T + LLE-rituximab, and the control groups, AdCAR-T, LLE-rituximab, and CD20-CAR-T are shown. F) Luciferase activity was quantified and plotted over time. Arrowhead indicates the termination of LLE-rituximab application. Comparison of BLI activity at day 44 revealed no difference between the groups conventional CD20CAR-T and AdCAR-T + LLE-rituximab (LLE-CD20 mAb) by Mann-Whitney test, p = .78. (BLI) bioluminescence. (n.s.) not significant.
