Table 1.
Scope of Alkenes in the DFA of Perfluorinated Esters
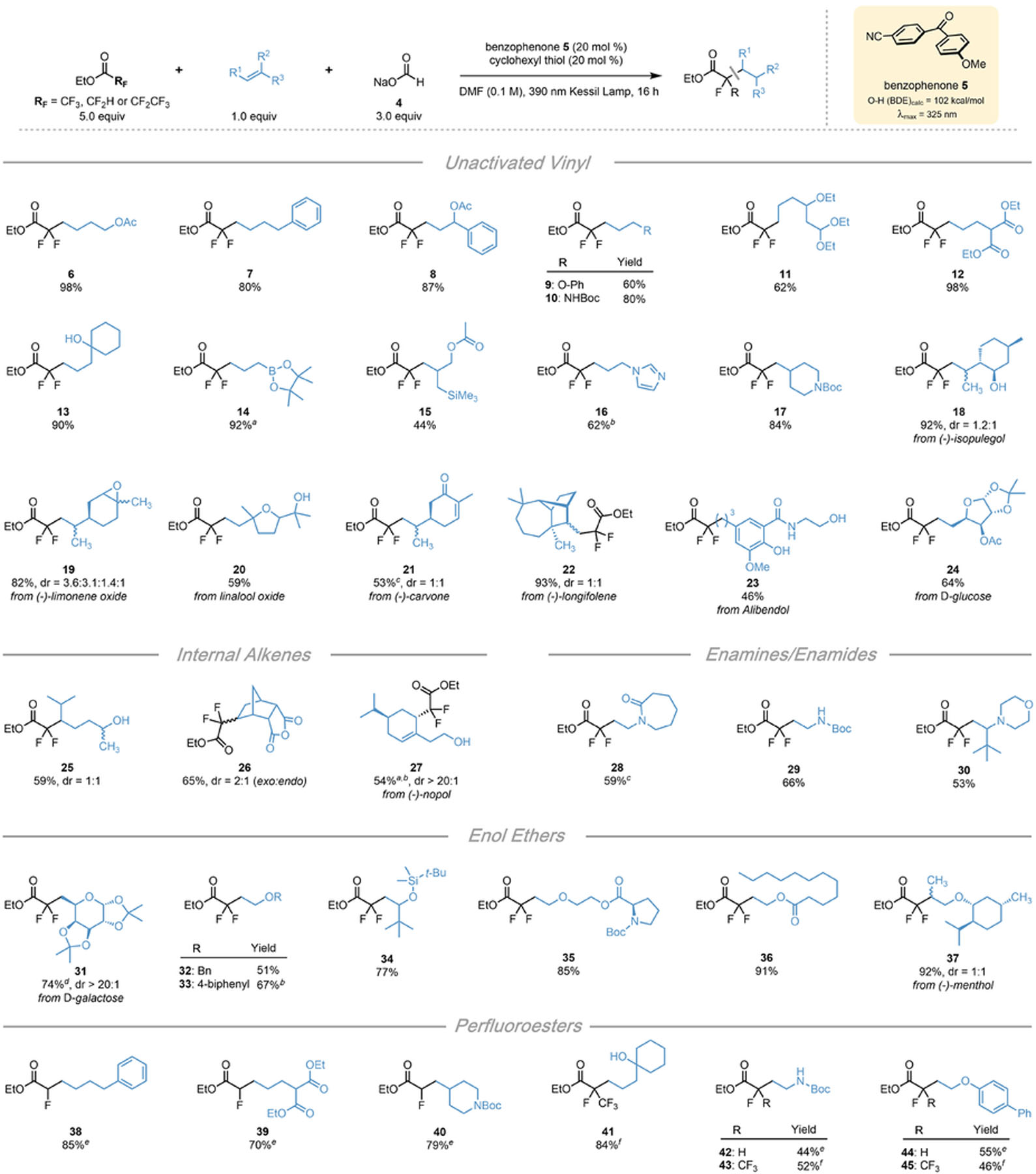
|
All values indicate the yield of the isolated product. Unless otherwise noted: alkene (1 equiv, 0.5 mmol), ethyl trifluoroacetate (5 equiv, 2.5 mmol), sodium formate (3 equiv, 1.5 mmol) benzophenone 5 (20 mol %, 0.10 mmol), cyclohexanethiol (20 mol %, 0.10 mmol), DMF (0.1 M), 16 h, irradiating with a 390 nm PR160 Kessil lamp.
H NMR yield of crude reaction.
Using 5 equiv of sodium formate and 10 equiv of ethyl trifluoroacetate.
Using 10 equiv of ethyl trifluoroacetate.
Isolated as the corresponding carboxylic acid.
Using ethyl difluoroacetate (10 equiv, 5.0 mmol) in place of ethyl trifluoroacetate with 48 h reaction time.
Using ethyl pentafluoropropionate (5 equiv, 2.5 mmol) in place of ethyl trifluoroacetate.
