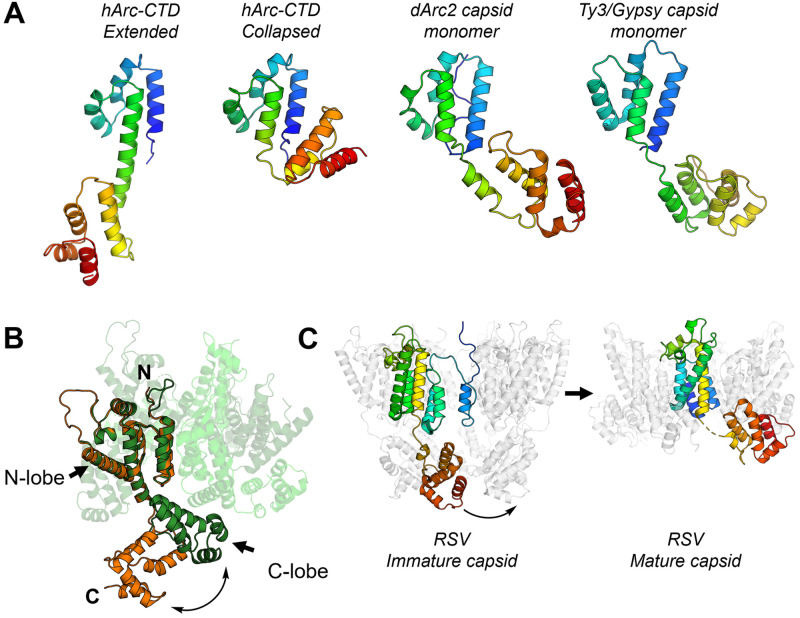
Fig 13. CTD hinge region flexibility is conserved in other LTR retrotransposons and retroviral CA domains.
A Comparison of the extended and collapsed crystal structures of the hArc-CTD, obtained in this study, with the dArc2 (6TAQ, [40]) and Ty3/Gypsy (6R24, [85]) capsid monomer reveal varying conformations of LTR retrotransposon capsid proteins. All are aligned on the N-lobe and shown in rainbow. See S13 Fig for an overall alignment of hArc-CTD and dArc2. B Comparison of the monomeric HIV CA, crystallised in complex with a Fab fragment (orange, 1E6J, [88]), and the CA in the capsid pentamer (3P05, [86]) reveal structural plasticity of the linker region, similar to that observed in the Arc-CTD. The HIV CA monomer is shown in orange and the capsid pentamer in green. The capsid protomers not aligned with the monomeric CA are shown in various shades of green transparent cartoons. C Structure of immature RSV viral particles (5A9E, [89]) and mature RSV capsid pentamers (7NO5, [90]) shows similar structural fluctuations in the process of capsid assembly.