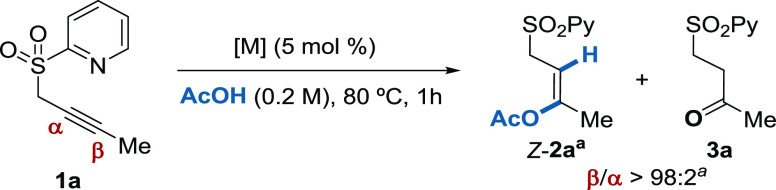
Table 1. Optimization Studies for the Acetoxylation of Substrate 1a.
| entry | catalyst | Z-2a/3aa | yield (%)b |
|---|---|---|---|
| 1 | Pd(OAc)2 | >98:2 | 86 |
| 2 | Pt(CH3CN)2Cl2 | 44:56 | 72 |
| 3 | AuCl(PPh3)/AgOTf | 62:38 | 66 |
| 4 | AgSbF6 | decomp | |
| 5 | Zn(ClO4)2·6H2O | nr | |
| 6c | Pd(OAc)2 | >98:2 | 41 |
| 7d | Pd(OAc)2 | >98:2 | 76 |
| 8 | PdBr2 | nr | |
| 9 | Pd(acac)2 | >98:2 | 10 |
| 10 | Pd(TFA)2 | nr | |
| 11 | PdCl2(CH3CN)2 | decomp | |
| 12 | none | nr | |
| 13 | Pd/Aue | 94:6 | 79 |
| 14 | Pd(OAc)2,H2Of | 86:14 | 81 |
| 15 | Pd/Aue,H2Of | 52:48 | 85 |
Determined by 1H NMR spectroscopy from the crude mixture.
Determined by 1H NMR using 1,3,5-trimethoxybenzene as an internal standard.
3 mol % Pd(OAc)2 was used.
Reaction performed at 60 °C during 5 h for full completion.
A combination of Pd(OAc)2 (5 mol %) and AuCl(PPh3)/AgOTf (5 mol %) was used as a catalyst.
Extra water (10% v/v) was added to the reaction mixture. nr: no reaction (starting material recovered).