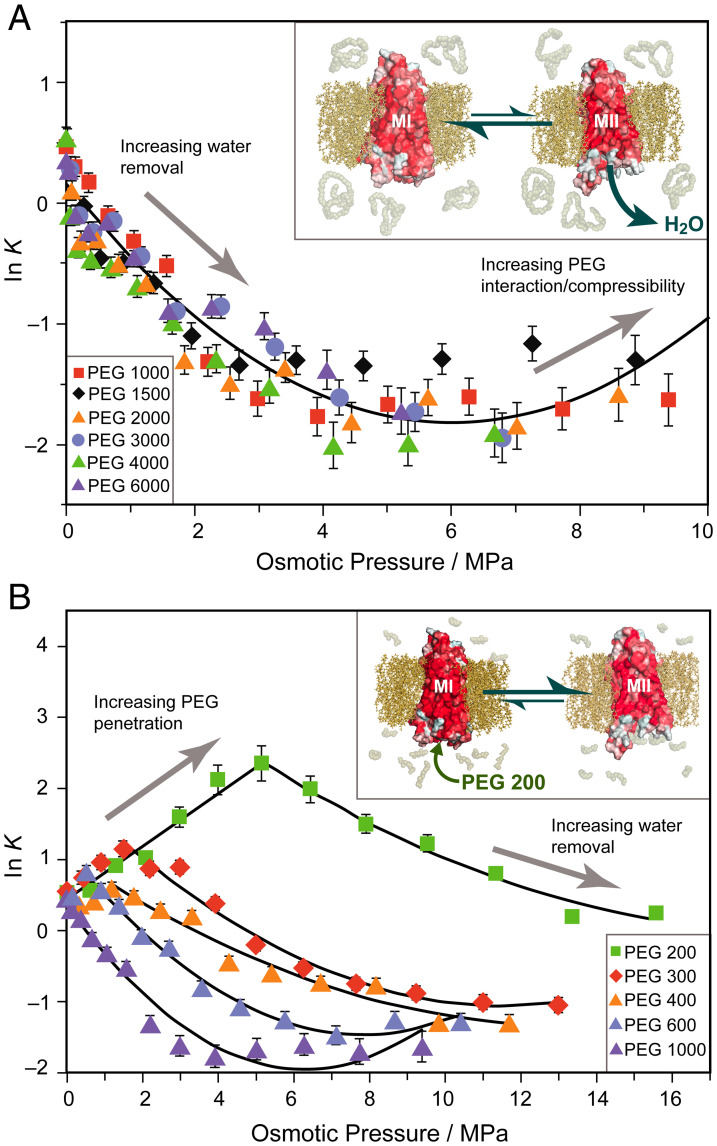
Fig. 3.
Large and small molar mass osmolytes affect rhodopsin differently and show emergence of a universal trend for excluded polymers. (A) Natural logarithm of the MI–MII equilibrium constant (K = [MII]/[MI]) has approximately second-order relationship to osmotic pressure of large PEGs. A universal trend arises for PEGs of Mr between 1,000 and 6,000 Da with the linear term proportional to change in hydration. Inset: Metarhodopsin equilibrium is shifted to the MI (closed) state by large polymer osmolytes which are entropically excluded and dehydrate the protein. (B) Initially, small osmolytes (PEG 200–PEG 600) forward shift ln K to the MII state. A saturation effect is observed beyond which the equilibrium is back shifted to MI resembling the large osmolyte behavior. As PEG size increases, the trend behaves more like the universal behavior. Inset: Small osmolytes such as PEG 200 penetrate the transducin binding cavity and stabilize the open active MII state until it is saturated with small PEGs.