Fig. 4.
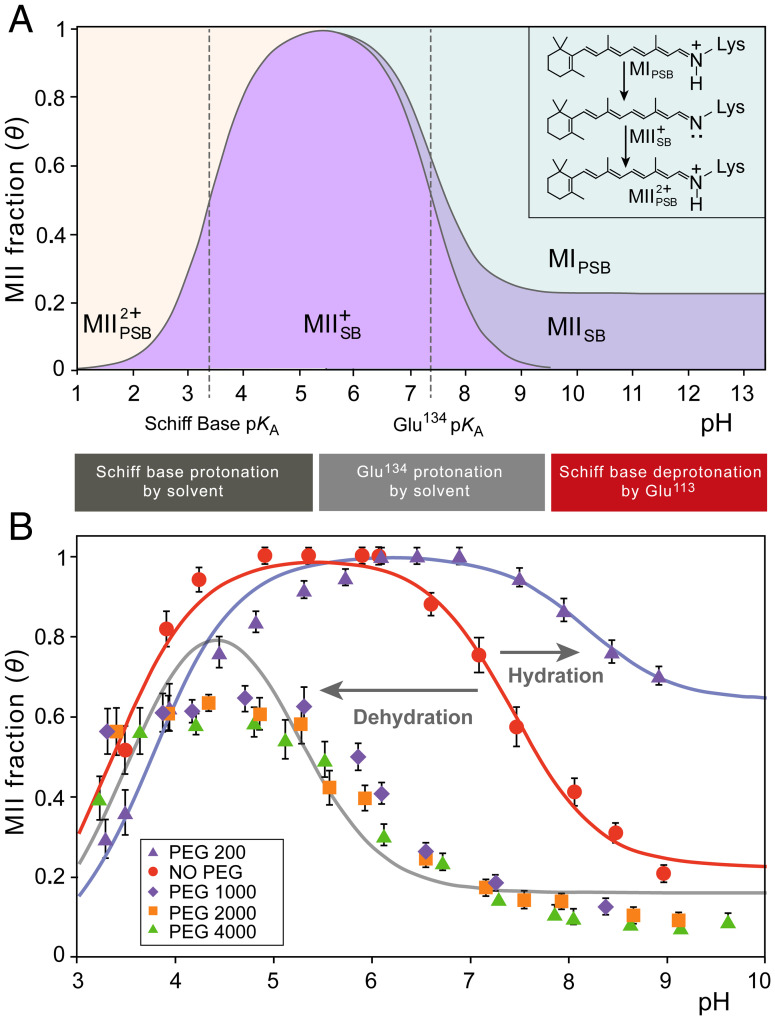
Osmotic pressure induces large shifts of pH-dependent activation of rhodopsin. (A) Influences of pH on rhodopsin are described by phenomenological Henderson-Hasselbalch equation involving two pKA values and an alkaline endpoint. The states are distinguished by having a protonated or deprotonated Schiff base (PSB or SB, indicated by a subscript), while a superscript indicates the charge relative to MI. The lower pKA (designated pKA,SB) reflects pH-dependent protonation of the retinal Schiff base (SB), which lowers the apparent MII fraction detected by UV-visible spectroscopy. The higher pKA value (designated pKA,Glu) reflects protonation of Glu134 in the E(D)RY motif to stabilize the fully active MII conformation. The alkaline endpoint at higher pH corresponds to MII substates that persist at higher temperatures even when Glu134 is fully deprotonated. (B) Applied osmotic stress stemming from large osmolytes (50% wt/wt at T = 15 °C) back shifts the rhodopsin activation titration curve from pKA = 7.4 to 5.2. At 30% wt/wt PEG 200 (T = 15 °C) the titration curve is maximally forward shifted to a pKA of 8.2 favoring the active MII state. Also observed is an osmolyte effect on the alkaline endpoint: small osmolytes stabilize the open MII conformation even when Glu134 is fully deprotonated, increasing the alkaline endpoint, whereas dehydrating large osmolytes decrease the deprotonated MII population and thus the alkaline endpoint.