Abstract
Gut dysbiosis is closely associated with the outbreak of inflammatory bowel disease (IBD) and psychiatric disorder. The Enterobacteriaceae population was higher in the feces of patients with inflammatory bowel disease (IBD-F) than in those of healthy control volunteers (HC-F). The Enterococcaceae and Lactobacillaceae populations were higher in the feces of IBD patients with depression (IBD/D+-F) vs. the feces of IBD patients without depression (IBD/D−-F). Therefore, we examined the effects of Klebsiella oxytoca, Escherichia coli, Cronobacter sakazakii, Enterococcus faecium, and Pediococcus acidolactici overpopulated in IBD/D+-F and their byproducts LPS and exopolysaccharide (EPS) on the occurrence of depression and colitis in mice. Oral gavages of Klebsiella oxytoca, Escherichia coli, and Cronobacter sakazakii belonging to Enterobacteriaceae, singly or together, caused dose-dependently colitis and depression-like behaviors in germ-free and specific-pathogen-free mice. Although Enterococcus faecium and Pediococcus acidolactici did not significantly cause colitis and depression-like behaviors, they significantly deteriorated Klebsiella oxytoca- or Escherichia coli-induced colitis, neuroinflammation, and anxiety/depression-like behaviors and increased blood LPS, corticosterone, and IL-6 levels. The EPSs from Enterococcus faecium and Pediococcus acidolactici also worsened Klebsiella oxytoca LPS-induced colitis, neuroinflammation, and depression-like behaviors in mice and increased the translocation of fluorescein isothiocyanate-conjugated LPS into the hippocampus. However, Bifidobacterium longum, which was lower in IBD/D+-F vs. IBD/D−-F, or its EPS suppressed them. In conclusion, Enterococcus faecium and Pediococcus acidolactici, known as a probiotic strain, and their EPSs may be a risk factor for the outbreak of depression and IBD.
Subject terms: Immunology, Microbiology
Introduction
Inflammatory bowel disease (IBD), including ulcerative colitis (UC) and Crohn’s disease (CD), is a chronic and recurrent disorders characterized by alternately repeating exacerbations and remissions1. Although obscure, the aetiology of IBD is thought to be the dysregulation of the mucosal immune system in the gut, resulting in an abnormal inflammatory response to environmental factors such as gut microbiota2,3. In patients with IBD, the prevalence of psychiatric disorders such as anxiety and depression is significantly higher than it is in healthy individuals4,5. Evidence in support of the close connection between IBD and psychiatric disorders stems primarily from animal and human studies5,6. Exposure to an acute psychological stress increases the secretion of the pro-inflammatory cytokines tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6 in both the blood and colon mucosa of patients with IBD7,8. In the Manitoba IBD Cohort Study population, 80% of patients with both IBD and anxiety disorder received the diagnosis of anxiety disorder more than 2 years before the IBD diagnosis9. Excessive exposure of mice to stressors, such as immobilization and pathogens, stimulates the secretion of adrenal hormones, such as cortisol, and immune cytokines, such as IL-1β and IL-6, via the activation of the hypothalamus − pituitary − adrenal (HPA) axis, resulting in the occurrence of colitis and gut dysbiosis accompanied by anxiety, depression, and memory impairment8,10–12. Antidepressant drugs attenuate colitis and anti-inflammatory drugs alleviate psychiatric disorders with colitis13,14. These findings suggest that the brain can bidirectionally communicate with the gut through the HPA and gut − brain axes.
The gut microbiota of healthy humans and animals consist of bacteria, viruses, fungi, archaea, and multicellular parasites15. Of these, commensal opportunistic pathogens, such as Escherichia coli and Klebsiella oxytoca, produce toxic byproducts, including lipopolysaccharide (LPS)16,17. Their overgrowth by stressors, such as antibiotics and immobilization stress, dysregulate gut immune homeostasis, resulting in gut inflammation through the excessive expression of proinflammatory cytokines8,11. The chronic stimulation of proinflammatory cytokines attenuates the expression of tight-junction proteins in the intestine, resulting in a leaky gut, as observed in patients with IBD18,19. The induction of leaky gut by gut inflammation accelerates the absorption of bacterial byproducts, such as LPS, into the blood and alters the gut microbiota composition, which is termed dysbiosis18,20,21. Patients with IBD exhibit a reduced gut microbial diversity with the increased Proteobacteria populations compared with healthy individuals22–24. The fecal microbiota transplantation (FMT) from patients with IBD causes the colitis in transplanted germ-free mice25,26. The FMT from mice with colitis also causes anxiety/depression with colitis in transplanted specific-pathogen-free mice10,11. The fecal microbiota of patients with inflammatory bowel disease patient feces (IBD-F) have the higher abundance of Enterobacteriaceae population and LPS levels compared to those of healthy control volunteers (HC-F)25. Enterococcaceae and Lactobacillaceae populations were higher in the feces of IBD patients with depression (IBD/D+-F) vs. the feces of IBD patients without depression (IBD/D−-F)25. The occurrence of colitis and psychiatric disorders triggered by intestinal environmental factors, such as antibiotics and pathogens, can be attenuated by treatment with commensal Lactobacilli and Bifidobacteria11,27. These findings suggest that the microbiota can communicate with the brain through the microbiota − gut − brain (MGB) axis, and that the interaction between gut microbiota may control the occurrence of gut inflammation and neuropsychiatric disorders.
Therefore, to understand the role of gut bacteria in the occurrence of IBD and depression, we examined the effects of gut bacteria overpopulated in IBD-F and their byproducts LPS and exopolysaccharide (EPS) on the occurrence of anxiety/depression and colitis in mice.
Results
Gut bacteria overpopulated in patients with IBD/D+ or IBD/D−
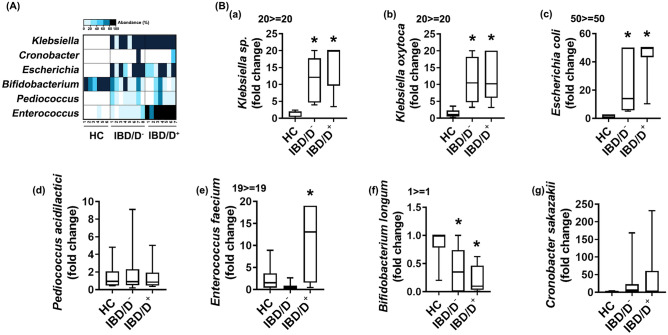
First, we analysed the live gut bacteria composition of HC-F, IBD/D–F, and IBD/D+-F using Enterobacteriaceae-selective DHL, Lactobacillaceae-selective MRS and Enterococcaceae-selective mEn, and Bifidobacteriaceae-selective BL agar plates (Fig. 1A). Klebsiella sp. (including Klebsiella oxytoca), Escherichia coli and Cronobacter sakazakii populations (in DHL agar plates) and Enterococcus sp. (including Enterococcus faecium) and Pediococcus acidilactici populations (in mEn and MRS agar plates) were highly detected in the IBD-F compared with HC-F. The Enterococcus sp. and Klebseilla sp. populations were the highest in the IBD/D+-F, followed by IBD/D−-F and HC-F, However, the Bifdiobacterium sp. population including Bifidobacterium longum was the highest in the HC-F, followed by the feces of patients with IBD/D− and IBD/D+. To confirm whether Enterobacteriaceae, Enterococcaceae, and Lactobacillaceae were associated with the occurrence of IBD and/or depression, we selected Klebsiella oxytoca and Escherichia coli, belonging to Enterobacteriaceae, Enterococcus faecium belonging to Enterococcaceae, and Pediococcus acidilactici belonging to Lactobacillaceae, which were highly detected in IBD/D+-F, and analyzed their populations in the HC-F and IBD-F by using qPCR (Fig. 1B). Their populations were more highly detected in the IBD-F compared with HC-F, while the Bifidobacterium longum population was lower in the IBD/D+-F vs. HC-F. The population of Enterococcus faecium was higher in the IBD/D+-F vs. IBD/D–F. However, Enterococcus faecium, Pedicoccus acidilactici, and Bifidobacterium longum did not exhibit hemolytic and gelatinolytic activities, like a well-known probiotics, while Enterococcus faecium was resistant to multi-antibiotics including ampicillin and streptomycin and produced biofilm (data not shown).
Figure 1.
The fecal microbiota composition of patients with IBD/D− or IBD/D+ and healthy individuals (HCs). (A) Effects on the levels of gut bacteria Klebsiella sp., Cronobacter sp., and Escherichia sp. grown in the DHL agar plate, Bifidobacterium sp. in the BL agar plate, and Pediococcus sp. and Enterococcus sp. in the mEn agar plate. (B) Effects on the levels of gut bacteria Klebsiella sp. (a), Klebsiella oxytoca (b), Escherichia coli (c), Pediococcus acidilactici (d), Enterococcus faecium (e), Bifidobacterium longum (f) and Cronobacter sakazekii (g), assessed by qPCR. Data are shown as box plots (HC n = 6; IBD/D− n = 8; IBD/D+, n = 7). Means with same letters are not significantly different (p < 0.05). (b,c), Kruskal–Wallis test (nonparametric test).
Effects of IBD/D+-F-abundant Enterobacteriaceae, Enterococcaceae, and Lactobacillaceae on the occurrence of colitis and anxiety/depression in mice
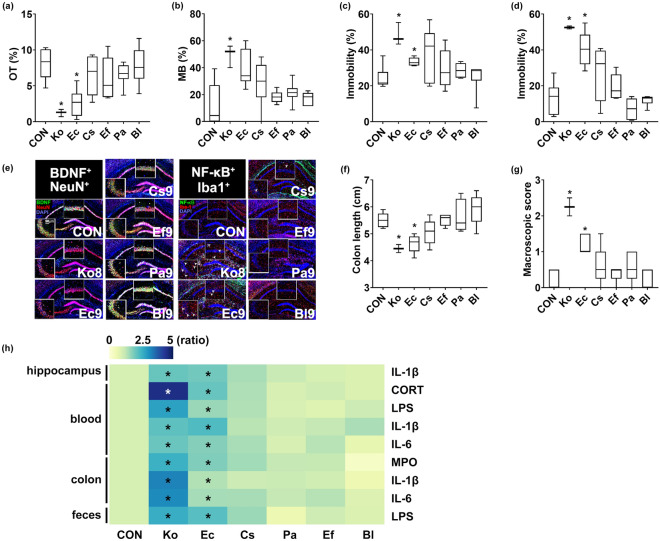
To understand the interactive effects of IBD/D+-F-abundant gut bacteria on the occurrence of colitis and anxiety/depression, we isolated Klebsiella oxytoca, Escherichia coli, Cronobacter sakazakii, Enterococcus faecium, Pediococcus acidilactici and Bifidobacterium longum and examined their effects on the occurrence of colitis and anxiety/depression in specific-pathogen-free mice (Fig. 2). Of these bacteria, Klebsiella oxytoca exhibited the highest lethal toxicity in specific-pathogen-free mice, followed by Escherichia coli. Oral gavage of Klebsiella oxytoca at doses of 1 × 108 and 1 × 109 CFU/mouse/day was lethal in 0% and 50% of mice within 5 days, respectively (Supplementary Figs. S1 and S2). Oral gavage of Escherichia coli, Cronobacter sakazakii, Enterococcus faecium, Pediococcus acidilactici or Bifidobacterium longum at a dose of 1 × 109 CFU/mouse/day did not kill any mice. Klebsiella oxytoca at doses of ≥ 1 × 107 CFU/mouse/day or Escherichia coli at doses of ≥ 1 × 108 CFU/mouse/day caused anxiety-like behavior in the elevated plus maze task (EPMT) and marble burying task (MBT) (Fig. 2a,b, Supplementary Figs. S1 and S2). Oral gavage of Klebsiella oxytoca at doses of ≥ 1 × 107 CFU/mouse/day, Escherichia coli at doses of 1 × 109 CFU/mouse/day caused depression-like behaviors in the tail suspension test (TST), and forced swimming test (FST) (Fig. 2c,d, Supplementary Figs. S1 and S2). Furthermore, their treatments increased the IL-1β expression, and the NF-κB+/Iba1+, LPS+/Iba1+, and IL-1R+ cell populations in the hippocampus and LPS levels in the blood while the BDNF+/NeuN+ cell population and claudin-5 expression decreased (Fig. 2e,h, Supplementary Figs. S1 and S2). However, oral gavage of Cronobacter sakazakii at a dose of 1 × 109 CFU/mouse/day did not cause anxiety-/depression-like behaviors.
Figure 2.
Effect of gut bacteria on the occurrence of anxiety/depression and colitis in mice. Effects on the time spent in open arms (OT) in the EPMT (a), marble-buried number in MBT (b), immobility time in the TST (c), and forced FST (d), and effects on the BDNF+/NeuN+ and NF-κB+/Iba1+ cell population in the hippocampus (e). Effects on the colon length (f) and macroscopic score (g). (h) Effects on the IL-1β, IL-6, LPS, myeloperoxidase (MPO), and corticosterone levels, indicated as compared to control group (CON: 1). Heatmap was generated using Plotly (https://chart-studio.plotly.com). Klebsiella oxytoca (Ko), Escherichia coli (Ec), Cronobacter sakazakii (Cs), Enterococcus faecium (Ef), Pediococcus acidilactici (Pa), or Bifidobacterium longum (Bl) at a dose of 1 × 109 CFU/mouse/day was orally gavaged in mice once a day for 5 days. Control mice (Con) were treated with vehicle (saline) instead of the gut bacterial suspension. Data are shown as box plots (n = 6). *p < 0.05 vs Con. Means with same letters are not significantly different (p < 0.05). All were analyzed using unpaired t test.
Klebsiella oxytoca at doses of ≥ 1 × 107 CFU/mouse/day or Escherichia coli at doses of ≥ 1 × 108 CFU/mouse/day also caused colitis in mice: they increased the stenosis score, myeloperoxidase activity, IL-1β and IL-6 expression, and the NF-κB+/CD11c+ cell population in the colon while the claudin-1 expression was decreased (Fig. 2f–h, Supplementary Figs. S1 and S2). They also increased corticosterone, IL-1β, IL-6, and LPS levels in the blood, as well as LPS level in the feces. However, Cronobacter sakazakii at a dose of 1 × 109 CFU/mouse/day weakly, but not significantly, caused colitis in mice.
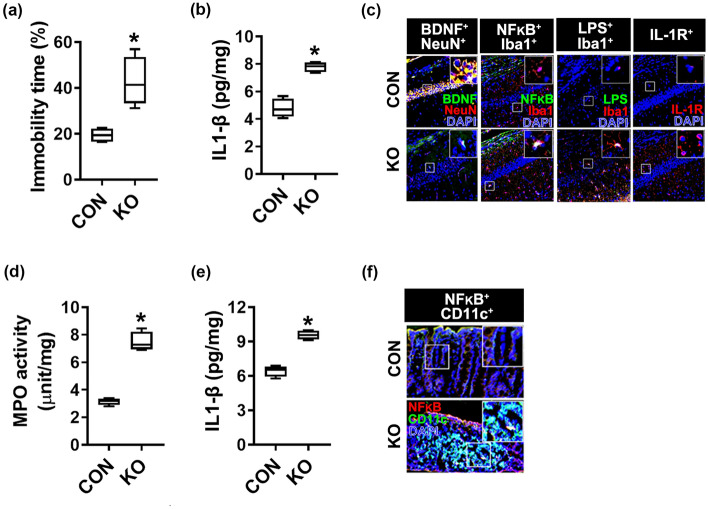
To understand whether gut microbiota-induced colitis and depression in between germ-free mice and specific-pathogen-free mice were different, we orally gavaged Klebsiella oxytoca into germ-free mice and examined its effects on the occurrence of colitis and depression in specific germ-free mice (Fig. 3, Supplementary Fig. S3). Oral gavage of Klebsiella oxytoca at a dose of 1 × 107 CFU/mouse/day caused colitis and anxiety/depression in germ-free mice. Klebsiella oxytoca caused dose-dependently depression-like behavior, increased NF-κB+/Iba1+ and LPS+/Iba1+ cell populations, upregulated IL-1β expression, and decreased the BDNF+/NeuN+ cell population in the hippocampus. Klebsiella oxytoca also upregulated IL-1β expression, myeloperoxidase activity, and the NF-κB+/CD11c+ cell population in the colon. The incidence of colitis and depression in germ-free mice by Klebsiella oxytoca at a dose of 1 × 107 CFU/mouse/day was similar to one treated at a dose of 1 × 108 CFU/mouse/day in specific-pathogen-free mice.
Figure 3.
Effect of Klebsiella oxytoca on the occurrence of depression and colitis in germ-free mice. Effect on the occurrence of depression-like behaviors (a) and hippocampal IL-1β level (b), BDNF+/NeuN+ (c), NF-κB+/Iba1+ (d), LPS+/Iba1+ (e), and IL-1R+ cell populations (f) in germ-free mice. Klebsiella oxytoca (KO, 1 × 107 CFU/mouse/day) were orally gavaged for 5 days in mice (n = 6, in specific-germ-free mice; n = 4, in germ-free mice). Control mice (CON) were treated with vehicle (saline) instead of the bacterial suspension. Data are shown as box plots. Means with same letters are not significantly different (p < 0.05). All were analyzed using unpaired t-test.
However, oral gavage of Enterococcus faecium, Pediococcus acidilactici, or Bifidobacterium longum at a dose of 1 × 109 CFU/mouse/day did not significantly cause colitis and anxiety-/depression-like behaviors in mice.
Interactive effects of Klebsiella oxytoca, Escherichia coli, and Cronobacter sakazakii on the occurrence of depression and colitis in mice
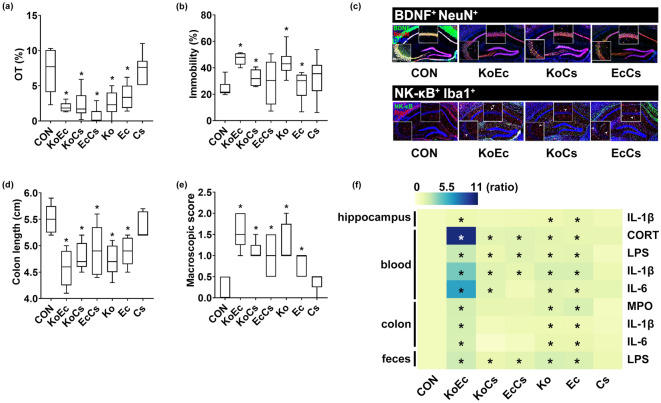
To understand the interactive effects of gut bacteria belonging to Enterobacteriaceae on the occurrence of colitis and depression, we examined the combined effects of two bacteria among Klebsiella oxytoca, Escherichia coli, and Cronobacter sakazakii (Fig. 4). These combinations additively increased the mortality and the occurrence of depression and colitis in mice. Of these combinations, those of Klebsiella oxytoca with Escherichia coli (KoEc, 1:1) and Klebsiella oxytoca with Cronobacter sakazakii (KoCs, 1:1) at a dose of 1 × 109 CFU/mouse killed 50% of mice (Supplementary Figs. S4 and S5). The combination of Escherichia coli with Cronobacter sakazakii (1:1) at a dose of 1 × 109 CFU/mouse did not kill mice. KoEc, KoCs, and EcCs caused anxiety-/depression-like behaviors in the EPMT and TST (Fig. 4a,b). They also increased IL-1β expression, and the NF-κB+/Iba1+, LPS+/Iba1+, and IL-1R+ cell populations in the hippocampus while the BDNF+/NeuN+ cell population and claudin-5 expression were decreased (Fig. 4c, Supplementary Figs. S4 and S5). They also increased corticosterone, IL-1β, IL-6, and LPS levels in the blood (Fig. 4f). KoEc also caused colitis most strongly, followed by KoCs and EcCs (Fig. 4d–f, Supplementary Figs. S4 and S5). They also increased LPS level in the feces.
Figure 4.
Combined effects of two bacteria belonging to Enterobacteriaceae on the occurrence of anxiety/depression and colitis in mice. Effects on the occurrence of anxiety/depression in the EPMT (a) and TST (b). Effects on the BDNF+/NeuN+ and NF-κB+/Iba1+ cell population in the hippocampus (c). Effects on the colon length (d) and macroscopic score (e). (f) Effects on the IL-1β, IL-6, LPS, MPO, and corticosterone levels, indicated as compared to control group (CON: 1). Heatmap was generated using Plotly (https://chart-studio.plotly.com). Among Klebsiella oxytoca (Ko), Escherichia coli (Ec), and Cronobacter sakazakii (Cs), two bacterial (KoEc, 1:1 of KO and Ec; KoCs, 1:1 of Ko and Cs; EcCS, 1:1 of Ec and Cs) combinations at a dose of 1 × 108 CFU/mouse/day were orally gavaged once a day for 5 days in mice. Control mice were treated with vehicle (saline) instead of gut bacterial suspension. Data are shown as box plots (n = 6). *p < 0.05 vs Con. All were analyzed using unpaired t test.
Interactive effects of Enterococcus faecium, Pediococcus acidilactici, and Bifidobcterium longum on the occurrence of colitis and anxiety/depression by Enterobacteriaceae
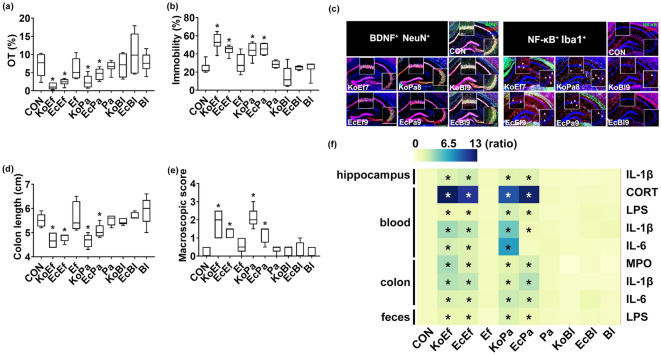
We examined the combined effects of Enterococcus faecium, Pediococcus acidilactici, and Bifdiobacterium longum on the occurrence of colitis and depression by Klebsiella oxytoca and Escherichia coli in mice (Fig. 5, Supplementary Figs. S6 and S7). Among the tested combinations, that of Klebsiella oxytoca with Pediococcus acidilactici (KoPa, 1;1) or Enterococcus faecium (KoEf, 1;1) at a dose of 1 × 109 CFU/mouse/day killed 100% of mice (Supplementary Fig. S4). These combinations at doses of ≤ 1 × 108 CFU/mouse/day did not kill any mice. Oral gavage of Klebsiella oxytoca with Pediococcus acidilactici (KoPa, 1;1) or Enterococcus faecium (KoEf, 1:1) caused anxiety-/depression-like behavior in the EPMT and TST (Fig. 5a,b). The combined treatment with Escherichia coli with Pediococcus acidilactici (EcPa, 1:1) or Enterococcus faecium (EcEf, 1:1) also caused anxiety-/depression-like behaviors. They also increased the NF-κB+/Iba1+, LPS+/Iba1+, and IL-1R+ cell populations and IL-1β expression in the hippocampus while the BDNF+/NeuN+ cell population and claudin-5 expression were decreased (Fig. 5c, Supplementary Figs. S6 and S7). Furthermore, they increased corticosterone, IL-1β, IL-6, and LPS levels in the blood (Fig. 5f, Supplementary Figs. S6 and S7). However, oral gavage of Bifidobacterium longum significantly protected the occurrence of anxiety/depression by Klebsiella oxytoca or Escherichia coli.
Figure 5.
Combined effects of Enterococcus faecium (Ef), Pediococcus acidilactici (Pa), or Bifidobacterium longum (Bl) with Enterobacteriaceae on the occurrence of anxiety/depression and colitis in mice. Effects on the occurrence of anxiety/depression in the EPMT (a) and TST (b). Effects on the BDNF+/NeuN+ and NF-κB+/Iba1+ cell population in the hippocampus (c). Effects on the colon length (d) and macroscopic score (e). (f) Effects on the IL-1β, IL-6, LPS, MPO, and corticosterone levels, indicated as compared to control group (CON: 1). Heatmap was generated using Plotly (https://chart-studio.plotly.com). Each two bacteria (KoEf, 1:1 of Ko and Ef; KoPa, 1:1 of Ko and Pa; KoBl, 1:1 of Ko and Bl; EcEf, 1:1 of Ec and Ef; EcPa, 1:1 of Ec and Pa; EcBl, 1:1 of Ec and Bl) combination at a dose of 1 × 107 (KoEf), 1 × 108 (KoPa), or 1 × 109 CFU/mouse/day (EcEf, EcPa, EcBl, Ef, Pa, and Bl) was orally gavaged once a day for 5 days in mice. Control mice were treated with vehicle (saline) instead of gut bacterial suspension. Data are shown as box plots (n = 8). *p < 0.05 vs Con. All were analyzed using unpaired t test.
Oral gavage of Enterococcus faecium or Pediococcus acidilactici also increased the occurrence of colitis by Klebsiella oxytoca or Escherichia coli (Fig. 5d-f, Supplementary Figs. S6 and S7). They increased colon shortening, myeloperoxidase activity, stenosis score, IL-1β and IL-6 expression, and NF-κB+/CD11c+ cell populations in the colon and LPS level in the feces, while the claudin-1 expression decreased. However, oral gavage of Bifidobacterium longum significantly protected the occurrence of colitis by Klebsiella oxytoca or Escherichia coli.
Effects of EPSs isolated from Enterococcus faecium, Pediococcus acidilactici, and Bifidobacterium longum on the occurrence of colitis and depression by LPS isolated from Klebsiella oxytoca
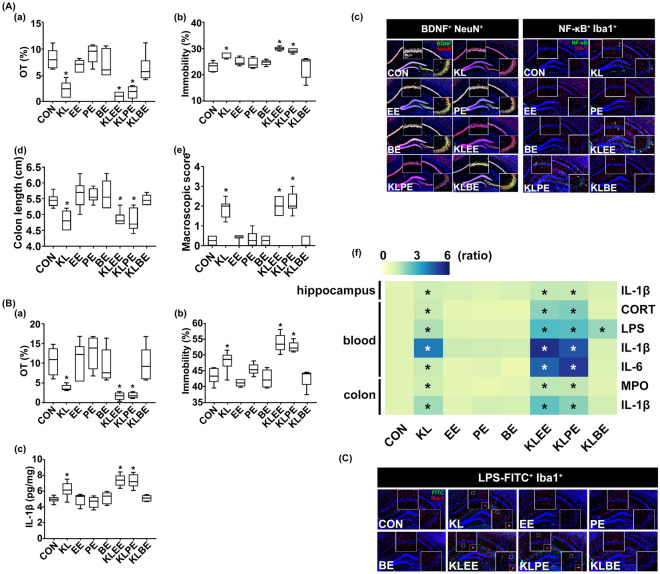
To understand the synergistically accelerative component(s) of Enterococcus faecium and Pediococcus acidilactici on the occurrence of anxiety/depression by Enterobacteriaceae anxiety/depression, we isolated EPSs and cytosolic fraction (CF) from Enterococcus faecium, Pediococcus acidilactici, and Bifidobacterium longum and LPS from Klebsiella oxytoca and examined the effects of EPSs and CFs on the occurrence of anxiety/depression in mice by LPS (Fig. 6, Supplementary Fig. S8). Oral gavage of EPSs or CFs did not cause anxiety-/depression-like behaviors and colitis, while that of Klebsiella oxytoca LPS significantly caused anxiety-/depression-like behaviors and colitis (Fig. 6A). The combined oral gavage of Enterococcus faecium EPS or Pediococcus acidilactici EPS with Klebsiella oxytoca LPS increased the occurrence of anxiety/depression than that of Klebsiella oxytoca LPS alone, while their CFs were not affect them (data not shown). Furthermore, EPSs increased LPS-induced NF-κB+/Iba1+ cell population and IL-1β and IL-6 expression and reduced LPS-suppressed BDNF+/NeuN+ cell population and claudin-5 expression in the hippocampus. They also synergistically increased LPS levels in the blood and myeloperoxidase activity and IL-1β expression in the colon, while the claudin-1 expression decreased in the colon. However, Bifidobacterium longum EPS significantly suppressed LPS-induced anxiety-/depression-like behaviors, hippocampal NF-κB+/Iba1+ cell population, and blood LPS level and induced LPS-suppressed hippocampal BDNF+/NeuN+ cell population and claudin-5 expression. Intraperitoneal injections of EPSs weakly, but not significantly, caused anxiety/depression and neuroinflammation, while that of LPS significantly caused anxiety-/depression-like behaviors and neuroinflammation (Fig. 6B). However, the combined injection of Enterococcus faecium EPS or Pediococcus acidilactici EPS with Klebsiella oxytoca LPS increased the occurrence of anxiety/depression more strongly than that of Klebsiella oxytoca LPS alone. However, Bifidobacterium longum EPS significantly suppressed the occurrence of anxiety/depression.
Figure 6.
Effects of gut bacteria EPS and LPS on the occurrence of anxiety/depression and colitis in mice. (A) Effects of orally gavaged EPS and/or LPS. Effects on the occurrence of anxiety/depression in the EPMT (a) and TST (b). Effects on the BDNF+/NeuN+ and NF-κB+/Iba1+ cell population in the hippocampus (c). Effects on colon length (d) and macroscopic score (e). (f) Effects on the IL-1β, IL-6, LPS, MPO, and corticosterone levels, indicated as compared to control group (CON: 1). Heatmap was generated using Plotly (https://chart-studio.plotly.com). (B) Effects of intraperitoneally injected EPS and/or LPS on the occurrence of anxiety/depression in the EPMT (a) and TST (b) and expression of IL-1β in the hippocampus (c). (C) Effects of EPSs on the translocation of FITC-conjugated LPS into the brain. Test agents (CON, vehicle [saline]; EE, 20 μg/kg (i.p.) or 20 mg/kg (p.o.) of Enterococcus faecium exopolysaccharide; PE, 20 μg/kg (i.p.) or 20 mg/kg (p.o.) of Pediococcus acidilactici exopolysaccharide; BE, 20 μg/kg (i.p.) or 20 mg/kg (p.o.) of Bifidobacterium longum exopolysaccharide; KL, 20 μg/kg (i.p.) or 20 mg/kg (p.o.) of Klebsiella oxytoca lipopolysaccharide; KLEE, 20 μg/kg (i.p.) or 20 mg/kg (p.o.) of Ko and Ef (1:1); KLPE, 20 μg/kg (i.p.) or 20 mg/kg (p.o.) of Ko and Pa (1:1); KLBE, 20 μg/kg (i.p.) or 20 mg/kg (p.o.) of Ko and Bl (1:1) were treated in mice once a day for 5 days. FITC-conjugated LPS was orally gavagaed daily for 2 days from 24 h after EPS or LPS conjugated without FITC was gavaged for 3 days. Control mice were treated with vehicle (saline) instead of gut bacterial suspension. Data are shown as box plots (n = 8). *p < 0.05 vs Con. All were analyzed using unpaired t test.
To confirm the accelerative effects of EPSs on the occurrence of anxiety/depression by Klebsiella oxytoca LPS, we orally gavaged fluorescein isothiocyanate (FITC)-conjugated LPS (fLPS) in mice (Fig. 6C, Supplementary Fig. S8). In the hippocampus of fLPS-treated mice, fLPS was detected. Furthermore, the simultaneous treatments of fLPS with EPSs increased fLPS amount and fLPS+/Iba1+ cell population in the hippocampus more than one without EPSs. However, Bifidobacterium longum EPS significantly reduced the fLPS+/Iba1+ cell population in the hippocampus.
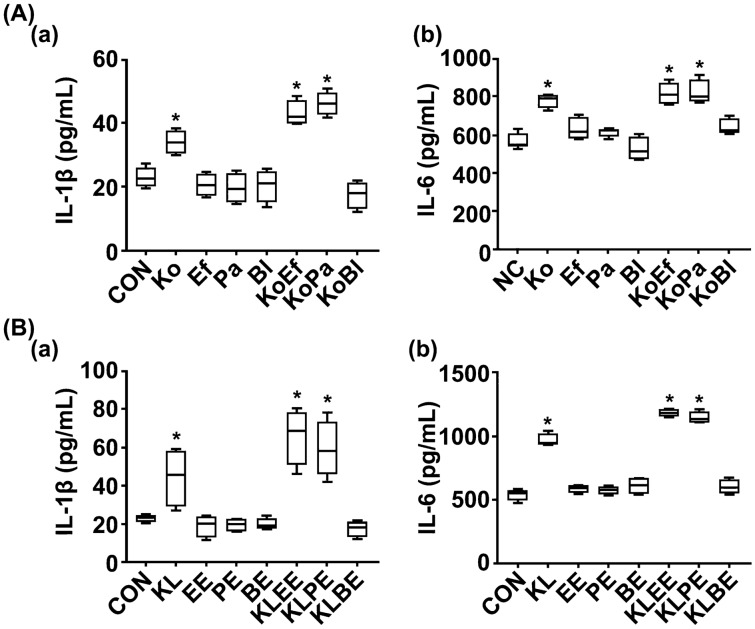
In the in vitro study, Klebsiella oxytoca and its LPS induced IL-1β and IL-6 expression in macrophages, while treatment with Enterococcus faecium, Pediococcus acidilactici, or Bifidobacterium longum was not affected (Fig. 7). Enterococcus faecium and Pediococcus acidilactici synergistically increased IL-1β and IL-6 expression, while Bifidobacterium longum suppressed it. EPS of Enterococcus faecium or Pediococcus acidilactici singly did not induce IL-1β and IL-6 expression, while the combinations of EPSs with LPS synergistically increased these cytokine expression. However, Bifidobacterium longum EPS suppressed LPS-induced IL-1β and IL-6 expression.
Figure 7.
Effects of Enterococcus faecium, Pediococcus acidilactici, and their exopolysaccharides on Klebsiella oxytoca- or its lipopolysaccharide-induced IL-1β and IL-6 expression and NF-κB activation in macrophages. (A) Effects of gut bacteria on IL-1β (a) and IL-6 expression (b) in macrophages. (B) Effects of gut bacterial LPS and EPS on IL-1β (a) and IL-6 expression (b) in macrophages. Test agents (CON, vehicle [saline]; Ef, 1 × 105 CFU/mL of Enterococcus faecium (Ef); Pa, 1 × 105 CFU/mL of Pediococcus acidilactici; Bl, 1 × 105 CFU/mL of Bifidobacterium longum; Ko, 1 × 105 CFU/mL of Klebsiella oxytoca; KoEf, 1 × 105 CFU/mL of Ko and Ef (1:1); KoPa, 1 × 105 CFU/mL of Ko and Pa (1:1); KoBl, 1 × 105 CFU/mL of Ko and Bl (1:1). KL, 100 ng/mL of Ko lipopolysaccharide (LPS); EE, 100 ng/mL of Ef expolysaccharide (EPS); PE, 100 ng/mL of Pa; BE, 100 ng/mL of Bl; KLEE, 100 ng/mL of Ef EPS with 100 ng/mL of Ko LPS; KLPE, 100 ng/mL of Pa EPS with 100 ng/mL of Ko LPS; KLBE, 100 ng/mL of Bl EPS with 100 ng/mL of Ko LPS) was treated in macrophages. The macrophages were treated with LPS (80 ng/mL) in the presence or absence of Ef, Pa, their EPSs, Ko, and/or its LPS for 20 h. Cytokine levels were assayed using ELISA kits. Data are shown as box plots (n = 6). *p < 0.05 vs Con. All were analyzed using unpaired t test.
Discussion
Gut inflammation and dysbiosis are closely associated with the occurrence of psychiatric disorders28,29. In particular, the high prevalence of commensal gut Protoebacteria including Enterobacteriaceae is closely associated with IBD, including UC and CD, and overexpression of gut bacterial LPS21,30. The induction of depression by stressors such as immobilization and antibiotics triggers colitis and increases the gut Proteobacteria population and bacterial LPS production in mice10,11. The populations of Alistipes (Bacteroidetes) and Enterobacteriaceae are overpopulated in patients with depression31. However, to date, none of these species/strains were convincingly shown to be associated with the occurrence of IBD and depression.
In the present study, we found that IBD-F exhibited a significantly higher abundance of Klebsiella oxytoca, Escherichia coli, and Enterococcus faecium and a lower abundance of Bifidobacterium longum than HC-F. The populations of Enterococcus faecium and Pediococcus acidilactici were higher in IBD/D+-F vs. IBD/D−-F. Among these bacteria, Klebsiella oxytoca caused colitis and anxiety/depression in SPF mice most potently, followed by Escherichia coli and Cronobacter sakazakii. Klebsiella oxytoca yielded the highest level of mortality in SPF mice. Interestingly, these bacteria at a low dose caused anxiety with colitis, while depression with colitis was caused at a high dose. The occurrence of depression and colitis after exposure to Klebsiella oxytoca was more severe in germ-free mice than in specific pathogen-free mice: the occurrence of depression by Klebsiella oxytoca at a dose of 1 × 107 CFU/mouse/day in germ-free mice was similar to that detected in specific-pathogen-free mice at a dose of 1 × 108 CFU/mouse/day. However, Enterococcus faecium, Pediococcus acidilactici, and Bifidobacterium longum did not significantly cause colitis and anxiety/depression. Högenauer et al. reported that Klebsiella oxytoca may be a causative organism of antibiotic-associated hemorrhagic colitis32. Jang et al. reported that antibiotics-induced Klebseilla oxytoca and its lipopolysaccharide caused colitis and anxiety in mice11. Jang et al. also reported that IS-induced Escherichia coli and its lipopolysaccharide caused colitis and depression in mice10. These results suggest that the occurrence of anxiety/depression with colitis by Enterobacteriaceae, particularly Klebsiella oxytoca, Escherichia coli, or Cronobacter sakazakii, may be dependent on their titers overgrown in the gastrointestinal tract.
The combination of two bacteria among Klebsiella oxytoca, Escherichia coli, and Cronobacter sakazakii additively caused colitis and anxiety/depression. The combination of Escherichia coli, Klebsiella oxytoca, or Cronobacter sakazakii belonging to Enterobacteriaceae with Pediococcus acidilactici or Enterococcus faecium synergistically increased the occurrence of colitis and depression caused by parenteral bacteria of Enterobacteriaceae. However, Pediococcus acidilactici or Enterococcus faecium did not caused colitis and depression. Of these combinations, KoPa, and KoEf most strongly caused depression and colitis, followed by EcEf and EcPa. However, when combined with Bifidobacterium longum, which is also known as a probiotic33, it significantly suppressed the occurrence of depression and colitis caused by Klebsiealla oxytoca, or Escherichia coli. Khan et al. reported that the populations of Bifidobacteria and Lactobacilli were higher in the IBD-F than in the HC-F34. Barandouzi et al. reported that the population of Bifidobacteriaceae, but not Bifidobacterium sp., was higher in the gut microbiota of patients with depression than in those of HCs35. Cheung et al. reported that patients with depression exhibited a lower abundance of Bifidobacteria and a higher abundance of Proteobacteria including Enterococcaceae compared with those in the HCs31. Although some of Pediococcus acidilactici or Enterococcus faecium are well-known GRAS (generally recognized as safe) probiotics36,37, some of them are virulent pathogens, which produce cytolysis38. Cambronel et al. and Scardaci, et al. reported that probiotic Enterococcus faecium or Enterococcus faecalis increased catecholamine-dependently biofilm production, resulting in the increased adhesion to the intestinal cells39,40. These results suggest that gut bacteria belonging to Enterobacteriaceae together can additively cause colitis and anxiety/depression, Enterococcus faecium (Enterococcaceae) and Pediococcus acidilactici (Lactobacillaceae) can severely elevate the occurrence of colitis and depression by Klebsiella oxytoca or Escherichia coli (Enterobacteriaceae). However, Bifidobacterium longum (Bifidobacteriaceae) may suppress Klebsiella oxytoca- or Escherichia coli-inducible colitis and anxiety/depression. This suggestion is supported by the previous report that oral gavage of ampicillin/amoxicillin increases the populations of gut Enterobacteriaceae belonging to Proteobacteria and Enterococcaceae, which exhibits multi-antibiotic resistance41, in mice, resulting in the occurrence of colitis and anxiety.
The content of LPS was higher in the IBD-F than in the HC-F. LPS content was weakly, but not significantly, higher in the IBD/D+-F than in the IBD/D−-F. Oral gavage of Klebsiealla oxytoca and Escherichia coli increased the fecal and blood levels and hippocampal NF-κB+/Iba1+ and LPS+/Iba1+ cell populations in mice, while hippocampal and colonic tight-junction protein expression and hippocampal BDNF+/NeuN+ cell population decreased. Interestingly, mice highly detected LPS levels in the blood showed depression-like behaviors rather than anxiety-like behaviors. Vogelzangs et al. reported that LPS more sensitively stimulate anxiety than depression. These results suggest that LPS can cause anxiety/depression and anxiety may be processed to depression LPS-dependently42. Oral gavage of Enterococcus faecium and Pediococcus acidilactici did not affect blood and fecal LPS levels. However, the combined treatment of Klebsiella oxytoca or Escherichia coli with them increased blood and fecal LPS levels and hippocampal NF-κB+/Iba1+ and LPS+/Iba1+ cell populations. However, the simultaneous treatment of Bifidobacterium longum with Klebsiella oxytoca or Escherichia coli also reduced blood and fecal LPS level and hippocampal NF-κB+/Iba1+ and LPS+/Iba1+ cell populations in the hippocampus compared with those treated with Klebsiella oxytoca or Escherichia coli alone. Jang et al. reported that the overexpression of fecal LPS after exposure to Escherichia coli significantly suppressed the expression of tight-junction proteins in the brain and colon11. Kim et al. reported that Escherichia coli treatment increased the NF-κB+/Iba1+ and LPS+/Iba1+ cell populations in the hippocampus and LPS levels in the blood and feces and decreased the BDNF+/NeuN+ cell population and tight-junction protein expression27. Lee et al. also reported that oral gavage of Escherichia coli and its LPS caused colitis and neuroinflammation in mice43. The induction of gut dysbiosis by high-fat diet and ampicillin causes leaky gut in mice11,44. IBD patients suffer from leaky gut45, a condition that accelerates the translocation of gut bacteria and their by-products such as LPS across the intestinal mucosa into the blood and brain, resulting in neuroinflammation46,47. These results suggest that Enterococcus faecium and Pediococcus acidilactici can elevate the occurrence of depression by Enterobacteriaceae such as Klebsiella oxytoca and Escherichia coli through the regulation of gut microbiota LPS-stimulated NF-κB activation and BDNF expression.
Exposure to EPS, not CF, of Enterococcus faecium or Pediococcus acidilactici with Klebsiella oxytoca LPS synergistically increased the occurrence of colitis, neuroinflammation, and anxiety/depression in mice, while Bifidobacterium longum EPS suppressed them. Moreover, these EPSs of Enterococcus faecium and Pediococcus acidilactici elevated the translocation of orally gavaged fLPS into the brain, while Bifidobacterium longum EPS suppressed it. The simultaneous stimulation of their EPSs with Klebsiella oxytoca LPS synergistically increased neuroinflammation and colitis in mice and proinflammatory cytokine expression in macrophages in vitro. The cell wall EPSs of Streptococcus sp. (Enterococcus sp.) and Bifidobacterium sp. have extremely varied structures. EPSs of some Enterococcus sp. cause inflammation in animals structure-dependently48, while the EPSs of Bifidobacteirum sp. modulate immune responses including inflammation49–51. In particular, the EPS of Bifidobacterium bifidum enhances proinflammatory immune response or induces immunosuppressive regulatory T cells52. Moreover, some gram-positive and gram-negative exopolysaccharides cause chronic brain inflammation in animals53. These results suggest that Enterococcus faecium, Pediococcus acidilactici, and/or their EPSs may elevate the LPS production of gut bacteria such as Klebsiella oxytoca and Escherichia coli, cause colitis, which can increase the translocation of gut bacterial LPS into the brain, resulting in the increased occurrence of anxiety/depression with neuroinflammation. However, the EPS of Bifidobacterium longum may inhibit colitis and neuroinflammation by the regulation of NF-κB activation, leading to the attenuation of anxiety/depression. Nevertheless, the relationship between the structures of EPSs and the occurrence of anxiety/depression and between their structures and biological activities remains unclear.
In conclusion, the interaction between gut microbiota Enterobacteriaceae, Enterococcaceae, Lactobacillaceae, and Bifidobacteriaceae may control the occurrence of IBD, neuroinflammation, and anxiety/depression through the regulation of gut microbiota LPS production and LPS-induced NF-κB activation-mediated BDNF expression. Enterococcus faecium and Pediococcus acidilactici EPSs can elevate the occurrence of anxiety/depression with IBD by Enterobacteriaceae. In particular, the occurrence of depression may be dependent on the gut dysbiosis-attributable overproduction of bacterial LPS and EPS. The imbalanced overgrowth of Enterococcus faecium and Pediococcus acidolactici, known as a probiotic strain, and imbalanced overproduction of their EPSs may be a risk factor for the outbreak of anxiety/depression in patients with IBD.
Methods
Volunteers
Volunteers, consisting of HCs (average age, 38.2 ± 2.2 years) and patients with IBD/D+ (average age, 46.4 ± 15.3 years) and IBD/D− (average age, 36.0 ± 12.6 years), were recruited from Kyung Hee University (Seoul, Korea) (Supplementary Table S1), as previously reported25. All patients with IBD enrolled in the study were > 13 years of age at the diagnosis of IBD, and all diagnoses were confirmed by previously established international criteria based on clinical, endoscopic, histopathological, and radiological findings54. The study protocol and informed consent forms for the stool collection were approved by the Committee for the Care and Use of Clinical Study of the Medical School of Kyung Hee University (IRB File No., KHUH 2018-03-006-018 and KHUH 2018-12-004-003). In a patient aged under 18, informed consent was obtained from a parent. All experiments were performed in accordance with relevant named guidelines regulations. We confirmed that informed consent was obtained from all participants before the initiation of the study and that all methods involving human participants were carried out in accordance with the ethical principles of the Declaration of Helsinki and the Korean Good Clinical Practice guidelines.
Animals
SPF C57BL/6 mice (male, 6 weeks old, 19–21 g) were purchased from Koatech Inc. (Seoul, Korea). Mice were kept in wire cages under a ventilated condition (20–22 °C, 50% ± 10% humidity, and 12-h/12-h light/dark cycle) and fed standard laboratory chow and water ad libitum. Germ-free C57BL/6 J mice (male, 18–21 g, 5 weeks old) were purchased from Clea Japan Inc. (Tokyo, Japan). Germ-free mice were housed in flexible film plastic isolators. All conditions were kept sterile in accordance with The Guidelines for Laboratory Germ-free Animals Care and Usage. Mice were used in the experiments after acclimation for 1 week. All animal experiments were approved by the Institutional Animal Care and Use Committee of Kyung Hee University [IACUC No., KUASP(SE)-18-033, KUASP(SE)-19-290, and KUASP(SE)-20-078] and were performed according to the NIH and University Guide for Laboratory Animals Care and Usage. This study additionally adheres to standards articulated in the ARRIVE guidelines.
Culture of gut bacteria
The fresh feces (0.5 g) of IBD patients and HCs were immediately collected, immediately suspended in 4.5 mL of general anaerobic medium (GAM, Nissui Pharmaceutical Inc., Tokyo, Japan) broth on ice, inoculated onto BL and DHL agar plates (Nissui Pharmaceutical Co. Ltd.), and cultured aerobically (for DHL agar plates) at 37 °C for 1 day or anaerobically (for BL and mEn agar plates) at 37 °C for 3 days27,43. The colonies grown in agar plates were inoculated into GAM semi-solid media. These bacteria were identified using Gram staining, 16S rRNA gene sequencing, and API kits, as previously reported10,21. For in vitro and in vivo experiments, gut bacteria were anaerobically cultured in the GAM broth at 37 °C (0.8–1.0 at 600 nm)10,21,42. Cultured bacteria were collected by centrifugation for 20 min at 5000g, and washed twice with saline. The collected cells (1 × 1010 CFU/mL) were suspended in saline.
Isolation of LPS and EPS
Pediococcus acidilactici, Enterococcus faecium and Bifidobacterium longum anaerobically cultured in the GAM broth at 37 °C were collected, washed with distilled water, suspended in distilled water, and sonicated according to the methods of Kim and Kobashi55. The sonicated suspension was centrifuged (10,000g, 4 ℃, 30 min). The supernatant faction was freeze-dried and used as cytosolic fraction (CF). EPSs were purified from their precipitates according to the method of Smitinont et al.56 Klebsiella oxytoca was anaerobically cultured in the GAM broth at 37 °C, and centrifuged for 20 min at 5000 g, and washed twice with saline. The collected cells (1 × 1010 CFU/mL) were suspended in distilled water. Its LPS was purified using a LPS extraction kit (iNtRON Biotechnolgy, Seongnam, Republic of Korea)42. FITC (F7250 Sigma, Aldrich)-conjugated LPS (FITC to LPS ratio, 0.2) was prepared as described by Park et al.57.
Culture of macrophage cells
Macrophages were isolated from the peritoneal cavity of mice intraperitoneally injected with sodium thioglycolate according to the method of Kim et al.27 Isolated macrophages were suspended in RPMI 1640 containing 10% fetal bovine serum and 1% antibiotics (RFA), seeded in 6-well plate, incubated at 37 °C for a day, and washed with RFA. The macrophages were treated in the presence or absence of test agents for 20 h. Cytokine levels were assayed using ELISA kits.
Treatment with gut bacteria in mice
To investigate whether gut microbiota could cause depression and/or colitis, we orally gavaged gut bacteria (1 × 106, 1 × 107, 1 × 108 or 1 × 109 CFU/mouse/day, suspended singly or together in saline) in mice once a day for 5 days and measured anxiety/depression-like behaviors for 3 day (Supplementary Fig. S9a,b).
To investigate whether the components of gut microbiota such as EPS, LPS, and CF could cause depression and colitis, Klebsiella oxytoca LPS (10 μg/kg/day for i.p. or 20 mg/kg/day for p.o.) in the absence or presence of EPG or CF from Pediococcus acidilactici, Enterococcus faecium, or Bifidobacterium longum (10 μg/kg/day for i.p. or 20 mg/kg/day for p.o.) were orally gavaged or intraperitoneally injected in mice once a day for 5 days (Supplementary Fig. S9c).
FITC-conjugated LPS (20 mg/kg/day) was orally gavaged daily for 2 days from 24 h after EPS or LPS conjugated without FITC was gavaged for 3 days. Anxiety-/depression-like behaviors were measured in the EPM and MB tasks, TST, and FST on the next day after EPS or LPS was treated. Mice were sacrificed 12 h after the final behavioral tasks. Colons and brains were removed and stored at – 80 °C for ELISA and immunoblotting.
Behavioral tasks
The EPM task was performed in a plus-maze apparatus (consisting of two open [30 × 7 cm] and two enclosed [30 × 7 cm] arm with 20-cm-high walls extending from a central platform [7 × 7 cm]), according to the method of Jang et al.10 MB task was assessed in a smooth, opaque plastic cage (30 × 35 × 13 cm) with a 5 cm layer of sawdust and 30 marbles on top of it (five rows or marbles regularly spaced at 2 cm away from the walls) according to Jang et al.10. The number of marbles buried for 30 min was counted. The TST was performed on the edge of a table, at 30 cm above it, for 5 min according to the method of Kim et al.27. Mice were judged to be immobile, when they did not move and hung passively. The FST was performed in a round transparent plastic jar (20 × 40 cm3) containing fresh water (25 °C) to a height of 25 cm for 5 min, according to Kim et al.27.
Immunofluorescence assay
The brains and colons were removed from mice perfused and post-fixed with paraformaldehyde, cytoprotected in 30% sucrose solution and cryosectioned. Sectioned tissues were immunostained according to the method of Lee et al.43.
Enzyme-linked immunosorbent assay (ELISA) and immunoblotting
Colon and brain tisusses were homogenized in the RIPA lysis buffer containing 1% phosphatase inhibitor cocktail and 1% protease inhibitor cocktail (RPP) on ice and centrifuged at 15,000×g at 4 °C for 15 min. The levels of cytokines in brain and colon tissues and corticosterone and cytokines in the plasma were assayed according to the method of Kim et al.27.
For the immunoblotting, the supernatants of the colon and cultured cell homogenates were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to nitrocellulose membrane27. Proteins were probed with antibodies, detected with horseradish peroxidase-conjugated secondary antibodies, and visualized with ECL detection kit.
Myeloperoxidase activity and limulus amebocyte lysate (LAL) assay
Myeloperoxidase activity was assayed according to the method of Jang et al.10. Blood and fecal endotoxin levels were assayed using an LAL assay kit (Cape Cod Inc., E. Falmouth, MA) according to the method of Jang et al.10.
Microbiota composition analysis
Bacterial genomic DNAs were extracted for the fresh feces of HCs, IBD patients, and feces-treated mice using a QIAamp DNA stool mini kit (Qiagen according to the method of Kim et al.27. Amplification of genomic DNA was performed using barcoded primers targeted the bacterial 16S rRNA V4 region gene and sequenced using Illumina iSeq 100 (San Diego, CA). Sequencing reads were deposited in the short read archive of NCBI under accession number PRJNA666980.
Quantitative real-time polymerase chain reaction (qPCR)
qPCR for gut bacteria was performed on the Rotor-Gene Q® thermocycler using DNA polymerase and SYBR Green I (Takara Bio Inc.: RR820A)27. PCR amplification reaction was carried out as follows: initial denaturation at 95 °C for 30 s, followed by 45 cycles of denaturation at 95 °C for 5 s, annealing at 55 °C for 30 min, and extension at 72 °C for 30 s27. Primers for qPCR are indicated in Supplementary Table S2.
Statistics
Experimental data are described as the mean ± SD using GraphPad Prism 8 (GraphPad Software, Inc., San Diego, CA, USA). Significant differences were analyzed using non-parametric ANOVA with Kruskal–Wallis test, one-way ANOVA with post-hoc Bonferroni's comparisons test, or unpaired t test, (p < 0.05).
Supplementary Information
Acknowledgements
We thank Dr. Young Joon Oh, a senior scientist in the World Institute of Kimchi, for the technical assistances for the maintenance and usage of the germ-free facility.
Author contributions
H.-M.J., C.K.L., H.-J.K., and D.-H.K. wrote manuscript text. H.-M.J. and M.-K.J. prepared Figs. 1, 2, and 7. J.-K.K., Y.-J.S. and D.-H.K. prepared Figs. 3, 4, 5 and 6. All authors reviewed the manuscript.
Funding
The present study was supported by the Medical Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science and ICT (NRF- 2017R1A5A2014768).
Competing interests
The authors declare no competing interests.
Footnotes
The original online version of this Article was revised: The original version of this Article contained an error in Figure 3, panel c, where the LPS+/Iba1+ and NF-κB+/Iba1+ images were duplicated.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Hyo-Min Jang, Jeon-Kyung Kim and Min-Kyung Joo.
Change history
8/10/2023
A Correction to this paper has been published: 10.1038/s41598-023-39969-8
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-13629-9.
References
- 1.Ananthakrishnan AN, et al. Environmental triggers in IBD: A review of progress and evidence. Nat. Rev. Gastroenterol. Hepatol. 2018;15:39–49. doi: 10.1038/nrgastro.2017.136. [DOI] [PubMed] [Google Scholar]
- 2.Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 2015;12:205–217. doi: 10.1038/nrgastro.2015.34. [DOI] [PubMed] [Google Scholar]
- 3.Zhang YZ, Li YY. Inflammatory bowel disease: Pathogenesis. World J. Gastroenterol. 2014;20:91–99. doi: 10.3748/wjg.v20.i1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byrne G, et al. Prevalence of anxiety and depression in patients with inflammatory bowel disease. Can. J. Gastroenterol. Hepatol. 2017;2017:6496727. doi: 10.1155/2017/6496727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graff LA, Walker JR, Bernstein CN. Depression and anxiety in inflammatory bowel disease: A review of comorbidity and management. Inflamm. Bowel Dis. 2009;15:1105–1118. doi: 10.1002/ibd.20873. [DOI] [PubMed] [Google Scholar]
- 6.Neuendorf R, Harding A, Stello N, Hanes D, Wahbeh H. Depression and anxiety in patients with inflammatory bowel disease: A systematic review. J. Psychosom. Res. 2016;87:70–80. doi: 10.1016/j.jpsychores.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Bonaz B, Sinniger V, Pellissier S. Anti-inflammatory properties of the vagus nerve: Potential therapeutic implications of vagus nerve stimulation. J. Physiol. 2016;594:5781–5790. doi: 10.1113/JP271539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mawdsley JE, Macey MG, Feakins RM, Langmead L, Rampton DS. The effect of acute psychologic stress on systemic and rectal mucosal measures of inflammation in ulcerative colitis. Gastroenterology. 2006;131:410–419. doi: 10.1053/j.gastro.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 9.Walker JR, et al. The Manitoba IBD cohort study: A population based study of the prevalence of lifetime and 12-month anxiety and mood disorders. Am. J. Gastroenterol. 2008;103:1989–1997. doi: 10.1111/j.1572-0241.2008.01980.x. [DOI] [PubMed] [Google Scholar]
- 10.Jang HM, Lee KE, Lee HJ, Kim DH. Immobilization stress-induced Escherichia coli causes anxiety by inducing NF-κB activation through gut microbiota disturbance. Sci. Rep. 2018;8:13897. doi: 10.1038/s41598-018-31764-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jang HM, Lee HJ, Jang SE, Han MJ, Kim DH. Evidence for interplay among antibacterial-induced gut microbiota disturbance, neuroinflammation, and anxiety in mice. Mucosal. Immunol. 2018;11:1386–1397. doi: 10.1038/s41385-018-0042-3. [DOI] [PubMed] [Google Scholar]
- 12.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gray MA, et al. Anti-TNFα therapy in IBD alters brain activity reflecting visceral sensory function and cognitive-affective biases. PLoS ONE. 2018;13:e0193542. doi: 10.1371/journal.pone.0193542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rahimi HR, Shiri M, Razmi A. Antidepressants can treat inflammatory bowel disease through regulation of the nuclear factor-κB/nitric oxide pathway and inhibition of cytokine production: A hypothesis. World J. Gastrointest. Pharmacol. Ther. 2012;3:83–85. doi: 10.4292/wjgpt.v3.i6.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamada N, Chen GY, Inohara N, Núñez G. Control of pathogens and pathobionts by the gut microbiota. Nat. Immunol. 2013;14:685–690. doi: 10.1038/ni.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fei, N., et al. Endotoxin producers overgrowing in human gut microbiota as the causative agents for nonalcoholic fatty liver disease. mBio11, e03263–3219 (2020). [DOI] [PMC free article] [PubMed]
- 17.Vasyurenko ZP, et al. Cellular and lipopolysaccharide fatty acid composition of the type strains of Klebsiella pneumoniae, Klebsiella oxytoca, and Klebsiella nonpathogenic species. Mikrobiol. Z. 2001;63:13–21. [PubMed] [Google Scholar]
- 18.Al-Sadi R, et al. Interleukin-6 modulation of intestinal epithelial tight junction permeability is mediated by JNK pathway activation of claudin-2 gene. PLoS ONE. 2014;9:e85345. doi: 10.1371/journal.pone.0085345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma TY, et al. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;286:G367–376. doi: 10.1152/ajpgi.00173.2003. [DOI] [PubMed] [Google Scholar]
- 20.Fuke N, et al. Regulation of gut microbiota and metabolic endotoxemia with dietary factors. Nutrients. 2019;11:2277. doi: 10.3390/nu11102277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jang SE, et al. Gastrointestinal inflammation by gut microbiota disturbance induces memory impairment in mice. Mucosal. Immunol. 2018;11:369–379. doi: 10.1038/mi.2017.49. [DOI] [PubMed] [Google Scholar]
- 22.Alam MT, Amos GCA, Murphy ARJ, Murch S, Wellington EMH, Arasaradnam RP. Microbial imbalance in inflammatory bowel disease patients at different taxonomic levels. Gut. Pathog. 2020;12:1. doi: 10.1186/s13099-019-0341-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gong D, et al. Involvement of reduced microbial diversity in inflammatory bowel disease. Gastroenterol. Res. Pract. 2016;2016:6951091. doi: 10.1155/2016/6951091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pittayanon R, et al. Differences in gut microbiota in patients with vs without inflammatory bowel diseases: A systematic review. Gastroenterology. 2018;158:930–946. doi: 10.1053/j.gastro.2019.11.294. [DOI] [PubMed] [Google Scholar]
- 25.Jang HM, et al. Transplantation of fecal microbiota from patients with inflammatory bowel disease and depression alters immune response and behavior in recipient mice. Sci. Rep. 2021;11:20406. doi: 10.1038/s41598-021-00088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weingarden AR, Vaughn BP. Intestinal microbiota, fecal microbiota transplantation, and inflammatory bowel disease. Gut Microbes. 2017;8:238–252. doi: 10.1080/19490976.2017.1290757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim JK, et al. Interplay between human gut bacteria Escherichia coli and Lactobacillus mucosae in the occurrence of neuropsychiatric disorders in mice. Front. Immunol. 2020;11:273. doi: 10.3389/fimmu.2020.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogers GB, et al. From gut dysbiosis to altered brain function and mental illness: Mechanisms and pathways. Mol. Psychiatry. 2016;21:738–748. doi: 10.1038/mp.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sochocka M, et al. The gut microbiome alterations and inflammation-driven pathogenesis of Alzheimer’s disease—a Critical review. Mol. Neurobiol. 2019;56:1841–1851. doi: 10.1007/s12035-018-1188-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mastuoka K, Kanai T. The gut microbiota and inflammatory bowel disease. Semin. Immunopathol. 2015;37:47–55. doi: 10.1007/s00281-014-0454-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheung SG, et al. Systematic review of gut microbiota and major depression. Front. Psychiatry. 2019;10:34. doi: 10.3389/fpsyt.2019.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Högenauer C, et al. Klebsiella oxytoca as a causative organism of antibiotic-associated hemorrhagic colitis. N. Engl. J. Med. 2006;355:2418–2426. doi: 10.1056/NEJMoa054765. [DOI] [PubMed] [Google Scholar]
- 33.Arboleya S, et al. Gut Bifidobacteria populations in human health and aging. Front. Microbiol. 2016;7:1204. doi: 10.3389/fmicb.2016.01204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khan I, et al. Alteration of gut microbiota in inflammatory bowel disease (IBD): Cause or consequence? IBD treatment targeting the gut microbiome. Pathogens. 2019;8:126. doi: 10.3390/pathogens8030126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barandouzi ZA, et al. Altered composition of gut microbiota in depression: A systematic review. Front. Psychiatry. 2010;11:541. doi: 10.3389/fpsyt.2020.00541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanchi H, et al. The genus Enterococcus: Between probiotic potential and safety concerns—An update. Front. Microbiol. 2018;9:1791. doi: 10.3389/fmicb.2018.01791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laranjo M, Potes ME, Elias M. Role of starter cultures on the safety of fermented meat products. Front. Microbiol. 2019;10:853. doi: 10.3389/fmicb.2019.00853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jett BD, Huycke MM, Gilmore MS. Virulence of enterococci. Clin. Microbiol. Rev. 1994;7:462–478. doi: 10.1128/CMR.7.4.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cambronel M, et al. Influence of catecholamines (epinephrine/norepinephrine) on Biofilm formation and adhesion in pathogenic and probiotic strains of Enterococcus faecalis. Front. Microbiol. 2020;11:1501. doi: 10.3389/fmicb.2020.01501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scardaci R, et al. Norepinephrine and serotonin can modulate the behavior of the probiotic Enterococcus faecium NCIMB10415 towards the host: Is a putative surface sensor involved? Microorganisms. 2022;10:487. doi: 10.3390/microorganisms10030487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller WR, Munita JM, Arias CA. Mechanisms of antibiotic resistance in enterococci. Expert. Rev. Anti Infect. Ther. 2014;12:1221–1236. doi: 10.1586/14787210.2014.956092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vogelzangs, et al. Cytokine production capacity in depression and anxiety. Transl. Psychiatry6, e825 (2016). [DOI] [PMC free article] [PubMed]
- 43.Lee KE, et al. The extracellular vesicle of gut microbial Paenalcaligenes hominis is a risk factor for vagus nerve-mediated cognitive impairment. Microbiome. 2020;8:107. doi: 10.1186/s40168-020-00881-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jeong MY, Jang HM, Kim DH. High-fat diet causes psychiatric disorders in mice by increasing Proteobacteria population. Neurosci. Lett. 2019;698:51–57. doi: 10.1016/j.neulet.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 45.D'IncàR MA. Intestinal permeability in inflammatory bowel disease: Pathogenesis, clinical evaluation, and therapy of leaky gut. Mediat. Inflamm. 2015;2015:628157. doi: 10.1155/2015/628157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Farhadi A, et al. Susceptibility to gut leakiness: A possible mechanism for endotoxaemia in non-alcoholic steatohepatitis. Liver Int. 2008;28:1026–1033. doi: 10.1111/j.1478-3231.2008.01723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fukui H. Increased intestinal permeability and decreased barrier function: Does it really influence the risk of inflammation? Inflamm. Intest. Dis. 2016;1:135–145. doi: 10.1159/000447252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spitznagel JK, et al. Modulation of complement fixation and the phlogistic capacity of group A, B, and D streptococci by human lysozyme acting on their cell walls. Infect. Immun. 1986;52:803–811. doi: 10.1128/iai.52.3.803-811.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pyclik M, et al. Bifidobacteria cell wall-derived exo-polysaccharides, lipoteichoic acids, peptidoglycans, polar lipids and proteins—their chemical structure and biological attributes. Int. J. Biol. Macromol. 2020;147:333–349. doi: 10.1016/j.ijbiomac.2019.12.227. [DOI] [PubMed] [Google Scholar]
- 50.Sarkar A, Mandal S. Bifidobacteria-Insight into clinical outcomes and mechanisms of its probiotic action. Microbiol. Res. 2016;192:159–171. doi: 10.1016/j.micres.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 51.Yu R, et al. Exopolysaccharide-producing Bifidobacterium adolescentis strains with similar adhesion property induce differential regulation of inflammatory immune response in Treg/Th17 axis of DSS-colitis mice. Nutrients. 2019;11:782. doi: 10.3390/nu11040782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Speciale I, et al. Bifidobacterium bifidum presents on the cell surface a complex mixture of glucans and galactans with different immunological properties. Carbohydr. Polym. 2019;218:269–278. doi: 10.1016/j.carbpol.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 53.Laman JD, et al. Bacterial peptidoglycan as a driver of chronic brain inflammation. Trends Mol. Med. 2020;26:670–682. doi: 10.1016/j.molmed.2019.11.006. [DOI] [PubMed] [Google Scholar]
- 54.Lennard-Jones JE. Classification of inflammatory bowel disease. Scand. J. Gastroenterol. 1989;170:2–6. doi: 10.3109/00365528909091339. [DOI] [PubMed] [Google Scholar]
- 55.Kim, D.H. & Kobashi, K. Induction and inhibition of novel sulfotransferase in a human intestinal bacterium, Eubacterium sp. A-44. Chem. Pharm. Bull (Tokyo). 39, 729–31 (1991). [DOI] [PubMed]
- 56.Smitinont T, et al. Exopolysaccharide-producing lactid acid bacteria streins from traditional thai fermented foods: Isolation, identification and exopolysaccharide characterization. Int. J. Food Microbiol. 1999;51:105–111. doi: 10.1016/S0168-1605(99)00094-X. [DOI] [PubMed] [Google Scholar]
- 57.Park KS, et al. Sepsis-like systemic inflammation induced by nano-sized extracellular vesicles from feces. Front. Microbiol. 2018;9:1735. doi: 10.3389/fmicb.2018.01735. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.