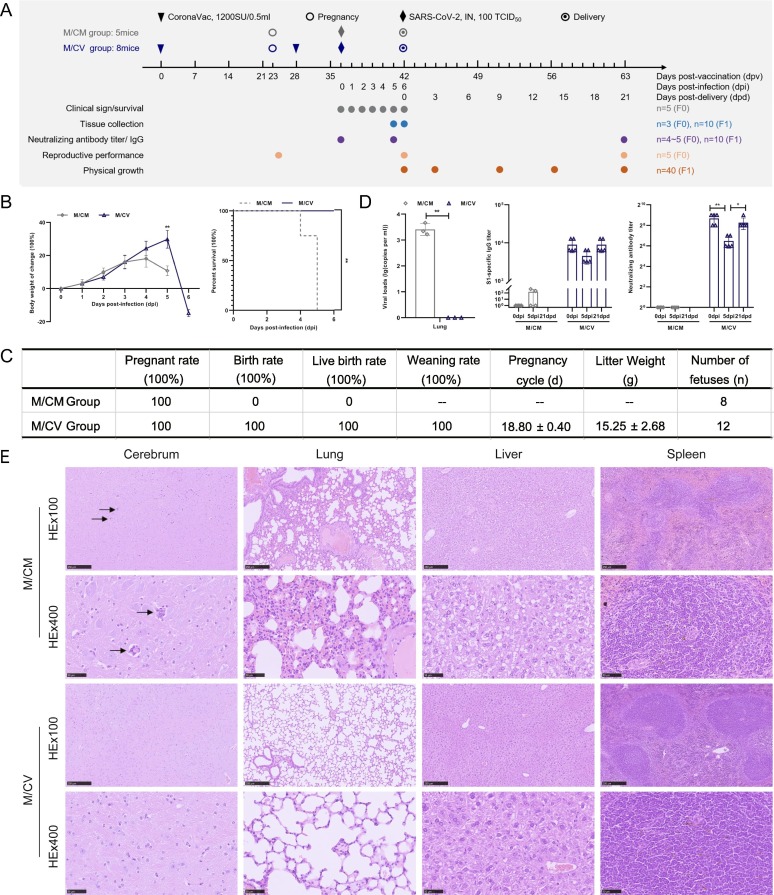
Fig. 3.
Clinical characteristics, reproductive performance, virus replication, antibody detection and histopathological characteristics in hACE2 pregnant mice infected with SARS-CoV-2. (A) Experimental design and sample collection. Thirteen mice were used in this study. Eight pregnant mice (M/CV group, n = 8) were inoculated with CoronaVac and challenged intranasally with SARS-CoV-2 at 100 TCID50. The control group (M/CM group, n = 5) were infected intranasally with the same dose of SARS-CoV-2. (B) Infected pregnant mice were observed for changes in body weight and percent survival at the indicated time points. (C) Reproductive performance indices. (D) The viral RNA loads of pregnant mice were quantified by RT-PCR at 5dpi; the titers of S1-specific IgG and NAb in hACE2 pregnant mice were also tested. (E) Histopathological characteristics of pregnant mice at 5 dpi were analyzed.