Abstract
Endocannabinoids mediate cellular functions and their activity is controlled by a complex system of enzymes, membrane receptors and transport molecules. Endocannabinoids are present in endometrium, a cyclical regenerative tissue requiring tightly regulated cellular mechanisms for maturation. The objective of this study was to investigate the gene expression of key elements involved in the endocannabinoid system across the menstrual cycle. RNA was isolated from endometrial tissue and genome-wide gene expression datasets were generated using RNA-sequencing. An a priori set of 70 genes associated with endocannabinoid system were selected from published literature. Gene expression across the menstrual cycle was analyzed using a moderated t test, corrected for multiple testing with Bonferroni’s method. A total of 40 of the 70 genes were present in > 90% of the samples, and significant differential gene expression identified for 29 genes. We identified 4 distinct regulation patterns for synthesizing enzymes, as well as a distinct regulation pattern for degradations and transporting enzymes. This study charts the expression of endometrial endocannabinoid system genes across the menstrual cycle. Altered expression of genes that control endocannabinoid may allow fine control over endocannabinoid concentrations and their influence on cellular function, maturation and differentiation as the endometrium matures through the menstrual cycle.
Subject terms: Physiology, Molecular medicine
Introduction
Endocannabinoids are small lipid-based molecules that play critical roles in regulating cellular functions. The most well characterised endocannabinoids are anandamide (AEA) and 2-arachdonoylglycerol (2-AG). Their production, concentration and cellular influence is mediated by a complex interaction of proteins that regulate their synthesis, transport, membrane binding, trafficking and degradation1. Collectively endocannabinoids and these proteins are termed the endocannabinoid system (ECS)2. Altered expression of any ECS component can lead to variation in endocannabinoid activity and alter their impact of cellular function.
Synthesis and degradation of AEA involves a number of enzymes. Four routes for AEA synthesis from NAPE have been reported and the most widely accepted pathway is the single-step, direct synthesis by the enzyme NAPE-PLD3–6. The remaining three pathways are two-step processes involving phospholipase C (PLC) and the protein tyrosine phosphatase N22 (PTPN22)7,8, phospholipase A2 (PLA2) and 2-lyso-phospholipase D (LysoPLD)9,10, and α/β hydrolase 4 (ABHD4) and glycerophosphodiester phosphodiesterase 1 (GDE1)11,12. Fatty acid amide hydrolase (FAAH) is the main enzyme responsible for degradation of AEA13,14. In addition AEA is subjected to oxygenation by a number of enzymes including cyclooxygenase-2 (COX-2)15,16, 5-, 12- and 15-lipoxygenase (5-/12-/15-LOX)17,18, and several cytochrome P450 monooxygenases (P450s) including CYP3A4, CYP4F2, CYP4X1, and CYP2D619,20. Two two-step synthetic pathways have been identified for the synthesis of 2-AG, and involve PLC21 followed by sn-1-diacylglycerol lipase (DAGL), or phospholipase A1 (PLA1) followed by lyso phospholipase C (lyso-PLC)22,23. The metabolism of 2-AG involves several enzymes including the main degrading enzyme monoacylglycerol lipase (MAGL)21,24, FAAH, ABHD6 and ABHD1225, COX-2 and LOXs16,26.
Endocannabinoids are found in many organs and tissues including the endometrium and influence cell migration27,28, proliferation29, survival30 inflammation31,32 and cellular differentiation33. The endometrium lines the uterus and is comprised of epithelial glandular structures, vascularised stroma and infiltrating immune cells. It is unique in that it undergoes cyclical growth, regeneration and shedding each month. Endometrial maturation during the menstrual cycle is controlled by female sex hormones, but also requires tightly regulated cellular responses to facilitate the cellular and structural changes. The proliferative phase is dominated by estrogen-driven cellular proliferation, vascularisation and immune stimulation while the secretory phase is dominated by progesterone mediated stromal cell decidualisation, vascular remodelling and immune cell modulation34. Regeneration of the endometrium following menses is mediated through adult stem and progenitor cells located in the basalis layer of the endometrium that proliferate, mature and differentiate across the menstrual cycle35,36. The consistent cyclical shedding and regrowth creates the monthly potential for errors in cell replication that may underlie both permanent, or transient endometrial abnormalities.
Endocannabinoids are abundantly expressed in the endometrium and influence endometrial cellular function. Synthetic endocannabinoid, methandamide induces endometrial stromal cell migration27, and AEA impairs both cellular proliferation and differentiation of an immortalised endometrial stromal cell line (St-T1b) and human decidual fibroblast from placenta, suggesting a crucial role in decidualisation37–39. AEA was also shown to influence migration in the immortalized human endometrial epithelial cell line HEC-1B28. Variations in concentrations of endocannabinoids may influence cellular proliferation and differentiation as the menstrual cycle progresses and the endometrium matures. Genes within the ECS are increasingly being recognised as targets to modulate endocannabinoid activity for multiple reproductive diseases including endometriosis, miscarriage, ectopic pregnancy, pre-eclampsia and endometrial cancer39,40. How these are altered across the menstrual cycle is not yet clear.
We hypothesised that the ECS plays a role in the cellular function of the endometrium, that they are regulated across the menstrual cycle and that this may have implications for how the endometrium matures. The goal of this study was to define an a priori set of ECS genes from the literature, analyse their mRNA expression in human endometrial tissue and determine variations in their expression across the menstrual cycle.
Results
ECS gene expression in the endometrium
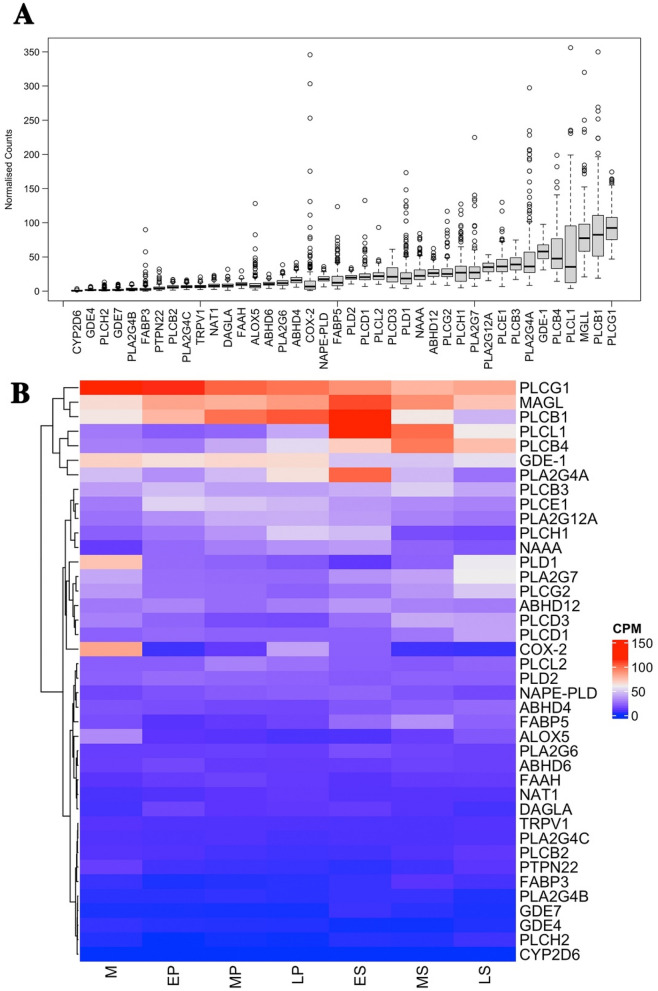
Of the 70 genes selected from a priori list for examination we found only 1 gene with no discernible expression in any endometrial samples (PLA2G2E), 64 were expressed in at least 1 sample with > 10 counts, 61 were expressed in 3 or more samples, 40 were expressed in greater than 90% of all samples (Supplementary Table S1). Synthesizing enzymes accounted for 45 of the investigated genes, 29 (64.4%) of which were expressed in > 90% of samples. Endocannabinoid degrading enzyme genes accounted for 12 of the investigated genes, of which 8 (66.7%) were expressed in > 90% of samples. Genes encoding transport proteins accounted for 7 of the investigated genes of which 2 were consistently expressed in > 90% of the samples. Finally, 6 genes for endocannabinoid membrane receptors were investigated and only one of these was expressed in > 90% of the samples. We focused the subsequent analysis on these 40 genes expressed in > 90% of the samples and their expression patterns across the menstrual cycle. The relative mean expression of each gene showed significant variation (Fig. 1A) and a heatmap analysis charting gene expression against the menstrual cycle indicated a large degree of this variability could be explained by changes associated with cycle stage (Fig. 1B). Five most expressed genes across the menstrual cycle are listed (Table 1).
Figure 1.
Gene expression of ECS genes in endometrium across the menstrual cycle. (A) Using 206 endometrial samples we found that of the 70 genes identified through the literature to function as part of ECS, 40 were expressed in at least 90% of the samples. The level of expression of each gene varied significantly between individuals and mean expression varied between genes. (B) A heat map analysis identifying the relative expression (cpm) of each gene across the menstrual cycle. The stage variation identified for some of these genes could be explained in part by differences that occurred in expression across the menstrual cycle. M = Menstrual, EP = Early proliferative, MP = Mid proliferative, LP = Late proliferative, ES = Early secretory, MS = Mid secretory, LS = Late secretory.
Table 1.
List of 5 most expressed genes across the menstrual cycle.
| Menstrual | Early proliferative | Mid proliferative | Late proliferative | Early secretory | Mid secretory | Late secretory |
|---|---|---|---|---|---|---|
| PLCG1 | PLCG1 | PLCG1 | PLCB1 | PLCB1 | PLCL1 | PLCG1 |
| COX-2 | MAGL | PLCB1 | PLCG1 | PLCL1 | PLCB4 | PLCB4 |
| PLD1 | PLCB1 | MAGL | MAGL | MAGL | MAGL | MAGL |
| GDE-1 | GDE-1 | GDE-1 | GDE-1 | PLA2G4A | PLCG1 | PLCL1 |
| MAGL | PLCE1 | PLCE1 | PLA2G4A | PLCG1 | PLCB1 | PLA2G7 |
5 highly expressed genes for each of the 7 menstrual stages with the most expressed gene at the top. The majority of the genes listed encode for synthesising enzymes of the endocannabinoid system with two exceptions of COX-2 and MAGL which encode for degrading enzymes.
ECS gene expression in women with and without endometriosis
Endometriosis is one of the most common endometrial pathologies occurring in 1 in 9 reproductive age women41,42 with suspected endometriosis the most common indication for surgery in this cohort. To determine whether endometrial pathologies, such as endometriosis, could influence endometrial ESC gene expression, we assessed the differences between women with and without endometriosis. Of the 206 samples collected 143 were confirmed endometriosis cases from visual inspection of the pelvis at laparoscopy and 63 were without evidence of endometriosis. Using the a priori set of selected genes and comparing the expression between women with and without endometriosis, no genes showed evidence of significant difference of expression when adjusted for multiple testing either compared together and taking into account menstrual cycle as a co-variate (Supplementary Table S2), or when comparing each cycle stage individually (Supplementary Table S3–S8). Previous studies on gene expression differences in these samples showed no genome-wide significance differences between women with and without endometriosis43,44. In subsequent analysis of expression across the menstrual stage therefore, we assessed all samples together, regardless of endometriosis status.
Gene expression of enzymes responsible for endocannabinoid production
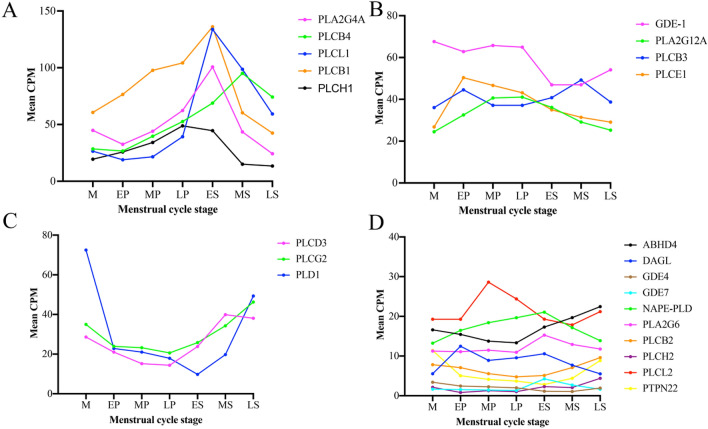
We performed 6 comparisons of gene expression between the 7 consecutive stages of the menstrual cycles, adjusting the p value to account for multiple testing of both multiple genes and menstrual cycle stage tests (Supplementary Table S9–S14). We identified a significant difference in 21 genes between the 7 consecutive stages of the menstrual cycle. Using a heatmap analysis we compared the expression of these genes across all stages and grouped those that showed similar levels of expression and pattern of regulation, identifying four distinct patterns of interest.
The first group contained five genes with a high median gene expression that increased consistently across the menstrual cycle from the EP stage and peaked in either the ES (PLA2G4A, PLCL1, PLCB1) or MS stage (PLCB4) followed by a decline in expression in the LS (Fig. 2A). Significant increases were observed for PLCL1 between the MP to LP stage (p = 0.004) and the LP to ES stage (p = 8.28 × 10−15), but was subsequently down regulated in the MS stage (p = 0.007). There was a significant increase in PLCB4 from the LP to ES stage (p = 0.007) with a further increase from the ES to MS stage (p = 0.003). A significant difference was recorded in the transition from the ES to MS stage for PLCB1 (p = 1.31 × 10−11). A significant decrease was observed for PLA2G4A in the transition from the MS stage to the LS stage (p = 1.31 × 10−11).
Figure 2.
The expression profile of the four distinct groups of endocannabinoid synthesizing enzymes. (A) Group 1 showed high gene expression (≈ 50–150 CPM) that increased across the proliferative stage and peaked during the early secretory to mid secretory stage. (B) The second group was characterised by median gene expression (≈ 20–80 CPM) that generally increased from the menstrual to early proliferative stage and then showed a gradual decline. (C) This group was characterised by a low- medium expression (≈ 10–80 CPM) that decreased in the early proliferative stage and subsequently increased in the secretory stage. (D) The fourth group was characterised by low gene expression (≈ 1–30 CPM) that showed only small changes across the stages. M = Menstrual, EP = Early proliferative, MP = Mid proliferative, LP = Late proliferative, ES = Early secretory, MS = Mid secretory, LS = Late secretory.
The second group of genes was characterised by a moderate mean expression with a gradual decrease across the cycle after initially peaking during the EP stage. An exception was observed with PLCB3 that showed a consistent expression across the cycle peaking in the MS stage (Fig. 2B). Significant increases in expression were observed during the transition from the M to EP stage for PLCE1 (p = 0.004) and PLA2G12A (p = 0.001) that also showed a significant decrease from the LP to ES (p = 0.007), as well as the ES to MS stage (p = 1.44 × 10−8). Significant decreases were also observed for GDE1 from the LP to ES stage (p = 7.81 × 10−8) and for PLCB3 in transition from the MS to LS stage (p = 0.003).
The third group was characterised by medium mean expression and that decreased directly after the menstrual stage and then subsequently increased again after the ES stage (Fig. 2C). Significant decreases in PLD1 were observed in the transition from the M to EP stage (p = 0.0005) and again from the LP to ES transition (p = 0.002). A significant increase was observed for PLCG2 (p = 0.005) and PLCD3 (p = 8.01 × 10−5) during the LP to ES transition. PLD1 began to increase in the MS stage (p = 0.0003) continuing into the LS stage (p = 2.8 × 10−8). Both PLCG2 (p = 0.005) and PLCD3 (p = 1.44 × 10−8) continued their increase during the early to mid-secretory stage.
The final group was characterised by low mean expression that showed multiple and varied expression changes across the EP to ES stage (Fig. 2D). Significant differences for the individual genes were observed between the M to EP stage for DAGL that was increased (p = 0.003) and PTPN22 that was decreased (p = 0.003). There was minimal regulation across the proliferative stage, however in the transition from the LP to ES stage there was a decrease in GDE4 (p = 5.48 × 10−5), PLCL2 (p = 0.007) and an increase in GDE7 (p = 2.93 × 10−11), ABHD4 (p = 0.002), PLCH2 (p = 0.0001) and PLA2G6 (p = 0.007). Significant changes also occurred in the ES to MS stage with a reduction in PLCH2 (p = 2.19 × 10−16), DAGL (p = 0.0004), GDE7 (p = 0.0009) and an increase in PLCB2 (p = 0.002) and PTPN22 (p = 0.001). NAPE-PLD decreased from the MS to LS (p = 0.003). Finally, PLCH2 (p = 0.003), PTPN22 (p = 0.0002) and GDE4 (p = 0.003) were all significantly increased in LS compared to the MS stage.
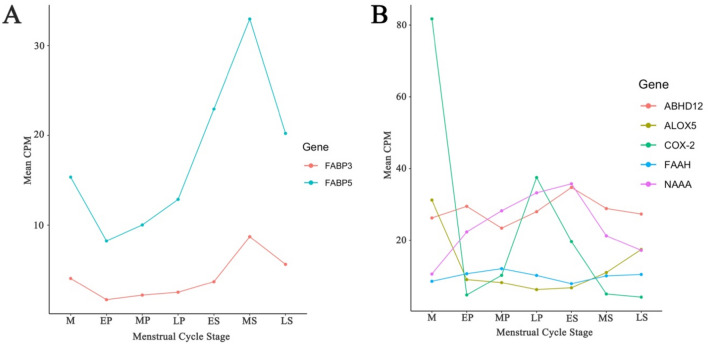
Gene expression encoding endocannabinoid transporters
We identified 7 genes known to encode fatty acid-binding proteins (FABPs) that transport endocannabinoids intracellularly. The largest changes were observed for FABP5 which was significantly upregulated from the LP to ES stage (p = 0.002) (Supplementary Table S12) with a subsequent down regulation from the MS to LS stage (p = 0.01) (Fig. 3A) (Supplementary Table S14). FABP3 was significantly upregulated in the transition from the ES to MS stage (p = 0.0005) (Supplementary Table S13).
Figure 3.
The expression profile of genes encoding ECS transport and degrading proteins. Transcription expression that showed a significant difference at any stage in the menstrual cycle were plotted against cycle stage. (A) For the transporting proteins moderate expression was observed for FABP5 and FABP3 that was significantly increased across the menstrual cycle. (B) ECS degrading enzymes had a moderate to low expression that were most commonly altered between the menstrual and early proliferative stage, or during the period from the late proliferative to mid secretory stage. Two genes ABHD12 and NAAA showed a consistent increase over the proliferative stage, after which there was decrease in expression. M = Menstrual, EP = Early proliferative, MP = Mid proliferative, LP = Late proliferative, ES = Early secretory, MS = Mid secretory, LS = Late secretory.
Gene expression of enzymes responsible for degradation of endocannabinoids
We identified expression of 8 of the 12 genes related to endocannabinoid degradation in endometrial tissue. Of these ALOX5 (p = 0.0005) and COX-2 (p = 0.008) were significantly down regulated and NAAA significantly upregulated in the transition from the M to the EP stage (p = 0.008) (Fig. 3B) (Supplementary Table S9). In the transition from the LP to ES stage, FAAH was decreased (p = 0.002) and ABHD12 was increased (p = 0.001) (Fig. 3B) (Supplementary Table S12). In contrast in the ES to MS stage FAAH was upregulated (p = 0.0003) and ABHD12 downregulated (p = 0.004) (Supplementary Table S13). Other significant changes from the ES to the MS stage included; NAAA (p = 5.04 × 10−7) and COX-2 (p = 1.15 × 10−6) that were downregulated and ALOX5 (p = 0.0002) that was upregulated.
Gene expression of endocannabinoid receptors
We assessed gene expression of 6 ECS membrane receptors across the menstrual cycle and between endometriosis status. Of these 6 genes we found that only one, TRPV1 was expressed in > 90% of the samples. It had a low but consistent expression and was not significantly regulated across the menstrual cycle or influenced by endometriosis status. The expression of all other membrane receptors occurred in < 90% of the samples. This suggests either a consistently low expression below read depth, or a binary on/off regulation. Based on the potential for binary regulation we determined the percentage of samples expressing membranes receptor genes in each menstrual stage (Fig. 4). For both CNR1 and CNR2 the number of samples with positive expression was between 70 and 100% for the various stages of the cycle, decreasing through the proliferative stage with a subsequent rise during the secretory stage. The changes however did not reach significance. GPR55 expression occurred in approximately 70% of samples, also decreasing through the proliferative stage reaching a minimum during the ES stage and subsequently increasing during the MS to LS stages. TRPA1 expression was observed only between the MP and MS stage and only in a low number of samples. Statistical analysis revealed there was no significant difference for any endocannabinoid receptor across the different stages of the menstrual cycle. Furthermore, no difference was observed in the percentage of samples expressing any of the membrane receptors when comparing endometriosis status and adjusting for menstrual cycle phase.
Figure 4.
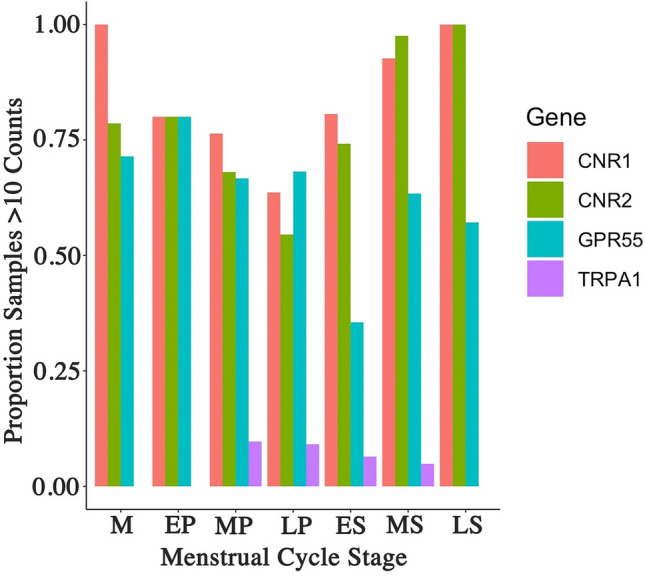
Logistic regression analysis of endocannabinoid receptor. Four of the five endocannabinoid receptor did not show consistent expression above > 90% in all samples. A logistic regression analysis examining the percentage of samples displaying a positive expression showed significant variation across the menstrual cycle for CNR1, CNR2 and GPR55. Only a small number of samples expressed TRPA1 and this was limited to the period between the mid proliferative to mid secretory stage. M = Menstrual, EP = Early proliferative, MP = Mid proliferative, LP = Late proliferative, ES = Early secretory, MS = Mid secretory, LS = Late secretory.
Correlation analysis with microarray
Microarray data was available on a subset of samples (n = 161). 35 of the 40 genes were detected in at least 50% of the microarray samples and included in the analysis to ensure sufficient pairwise comparisons to estimate an accurate correlation. Using the ‘cor.test' function in R we estimated the Pearson correlation between normalized and batch corrected counts from RNA-Seq (log2-CPM) and microarray (log2-normalised signal) for each gene across the 161 samples. All 35 genes detected were positively correlated with a mean correlation value of 0.62 confirming a robust measurement of gene expression using the RNA-seq technique, despite the differences between microarray and RNA-seq including the inability of microarray to measure all transcript variations and different detection thresholds (Supplementary Table S15).
Discussion
Endocannabinoids, naturally synthesized lipids are produced in abundance in the female reproductive tract45. They are present in normal endometrium and significantly influence cellular maturation and function46. Modulation of the ECS system occurs through enzymes regulating production, transport and degradation of the endocannabinoid signaling molecules. Understanding enzyme expression may provide insights into how endocannabinoid levels are modulated in endometrium. Utilizing genome-wide gene expression data of endometrial samples we performed a comprehensive analysis of ECS gene expression during the menstrual cycle. We identified significant regulation across the menstrual cycle for many genes and identified distinct patterns of regulation that strongly support alteration in endocannabinoid activity across the menstrual cycle that could be both a consequence of, or contribution to the changes that occur during cyclical endometrial maturation.
Enzymes capable of synthesizing endocannabinoid production were the most abundant in the endometrium, and also showed the greatest level of menstrual cycle regulation. The majority of gene expression changes occurred during the transition from the late proliferative to early secretory stage and continued across the secretory stage. The transition from proliferative to secretory stage is characterized by ovulation and an increase in the production of progesterone. Progesterone levels also fluctuate during the secretory stage by increasing up to the mid secretory stage and decreasing during the late secretory stage34. Gene expression changes across the cycle suggest an important role of steroid hormones in ECS modulation, in particular progesterone. Previous studies have reported progesterone is involved in the maintenance of endocannabinoid levels of the endometrium47. Progesterone was found to induce CB1 expression in endometrial stromal cells31, and to activate FAAH promoter decreasing AEA levels in human T lymphocytes48. In mice, progesterone has been shown to down-regulate uterine NAPE-PLD expression, possibly leading to a decrease in AEA levels49. Although in contrast, one study reported no correlation between plasma levels of AEA and progesterone in normal cycling women50.
The most abundant endocannabinoids in the endometrium, AEA and 2-AG, are predominantly synthesized via NAPE-PLD and DAGL respectively1, representing two of the most important enzymes in the ECS. We found both enzymes were downregulated during the transition from menstrual to early proliferative stage and then subsequently upregulated from early to mid-secretory and again from the mid to late secretory stage. This suggests an increased capacity for endocannabinoid production as the menstrual cycle progresses. This increased capacity may not be linked to progesterone as it decreases towards the end of the cycle, a period where endocannabinoid production capacity is still increasing. A previous study reported NAPE-PLD immunoreactivity was increased in the menstrual, early proliferative and late secretory glands with its lowest levels in the early secretory phase51. In a mouse model progesterone was reported to decrease NAPE-PLD expression49. The increase in these enzymes and the potential increase in the endocannabinoids may have implications for cellular function and differentiation during the menstrual cycle in humans and rodents.
A number of other enzymes can regulate endocannabinoid production through alternate pathways. One such group includes the many isoforms of phospholipase C (PLC). PLCs selectively catalyze the degradation of phosphatidylinositol 4,5 bisphosphate resulting in soluble inositol-1,4,5-triphosphate (IP3) and membrane delimited 1,2 diacylglycerol (DAG)52. DAG is an important precursor for 2-AG53. We observed multiple, but distinct patterns of PLC regulation across the menstrual cycle, particularly during the transition from the proliferative to secretory stage. This regulation would impact DAG expression subsequently influencing 2-AG production. Whether each of these isoforms has similar efficacy in the metabolism of IP3 and the subsequent production of DAG in the endometrium is unclear from this study.
Endocannabinoids continue to exert their activity until degraded. A number of enzymes responsible for the degradation of endocannabinoids, including FAAH, MGLL and ABHD12 were also significantly downregulated during the transition from the late proliferative to early secretory stage. A down regulation of these enzymes would potentiate the ability of endocannabinoids to continue signaling. Of these enzymes FAAH is of particular interest. FAAH is a principal enzyme in the degradation of AEA and previous evidence suggests a relationship to progesterone54,55. FAAH has previously been implicated in the survival of endometrial stromal cells from ectopic endometriosis lesions56 and has received significant interest as a pharmacological target, modulating pain through both peripheral and central mechanisms57. Physiological concentrations of progesterone stimulated FAAH activity in human lymphocytes, decreasing AEA levels48,54,58, while in a mouse model progesterone was reported to decrease uterine FAAH activity59. Progesterone levels and FAAH expression have been correlated during the menstrual cycle60, in agreement with the finding that progesterone up-regulates FAAH gene expression48,58. Combined with the regulation of the PLCs during this period, this suggests a regulation of endocannabinoid turnover during the transition between menstrual cycle stages that is tightly regulated.
Additional factors, other than their production and inhibition, can influence endocannabinoids concentrations and their activity. Intracellular degradation relies on lipophilic endocannabinoid reaching the intracellular enzymes. One of the most significant changes we observed in this gene set was the regulation of FABP5 across the menstrual cycle. FABP5 is a fatty acid binding protein that transports endocannabinoids through the aqueous cellular environment, preferentially directing it towards FAAH61. FABP5 was also significantly downregulated during the transition from the late proliferative to the early secretory stage. A reduction in the transport towards the degrading enzyme could potentiate the presence of the endocannabinoids during this period and may contribute to cellular and inflammatory changes34,62 that enables the regeneration of the endometrium in the early and mid-secretory phase in non-conceptive cycles.
Although we included an extensive list of genes encoding endocannabinoid receptors, including CNR1, CNR2, TRPV1, TRPA1, GPR55, and GPR119 we found that only one of these (TRPV1) was consistently expressed in > 90% of the samples. Both CNR1 and CNR2 were previously shown to be expressed in the endometrium with variation in expression between women with and without endometriosis31. We found expression in the majority of samples, but not sufficient to reach the > 90% cut off. Analysis of gene expression for the remaining receptors as a binary variable found changes across the menstrual cycle. Although we did not find any that reached significance after multiple testing, this may be related to the power of the sample size, or the complex milieu of individual cell types and maturing cell states that exist within the endometrium at any point in time. The results showed non-significant changes in number of samples expressing CNR1, CNR2 and GPR55 during the late proliferative to early secretory period. While CB1 receptor (the protein encoded by the CNR 1 gene) was found to inhibit human decidualization and stimulates apoptosis38, studies on CB1 expression in the epithelial glands found no significant regulation across the menstrual cycle51, and CB2 expression in stromal cells was similar between the proliferative and the secretory phase30. Previous studies have also reported that progesterone exerted minimal effects on CB1 expression in lymphocytes48,54,58. It is therefore likely that the lack of significant changes in the expression of these receptors across samples and across the menstrual cycle indicates they may not be significantly regulated by reproductive hormones in endometrial tissue. The ability of these genes to be switched on or off may reflect the selective use in patients, depended on local requirements and provide the ability to fine tune the activity of the ECS.
From our study cohort, 143 (69.4%) were diagnosed with endometriosis. Previous studies on the ECS in women with endometriosis have shown conflicting results. One reported no significant difference in NAPE-PLD and FAAH expression in the endometrium of patients compared to women without endometriosis throughout the menstrual cycle63. Another study reported no difference in CB1 protein expression during the proliferative phase between patients with and without endometriosis63, whereas lower levels of CNR1 and the protein CB1 have been reported in endometrial tissue from women with endometriosis compared to controls regardless of the cycle phase31. No difference in TRPV1 expression between women with and without endometriosis throughout the menstrual cycle has also been reported63. In this current study, when accounting for menstrual stage and multiple testing, we found no significant difference in any of the ECS genes investigated between women with and without endometriosis. Our data therefore correlates with previously published data that shows the ECS is not significantly dysregulated in eutopic endometrial tissue from patients with endometriosis.
This is the largest number of endometrial samples utilized to investigate gene expression of the ECS through menstrual cycle, and samples were assigned to detailed 7 menstrual stages following the Noyes criteria. However, the distribution of samples across the menstrual stages varied and the results should be interpreted with caution when the sample sizes for menstrual stages were small. While we were able to analyze the majority of the ECS at the gene level with RNA-seq data, we are yet to translate this to protein. Difference in gene and protein expression are commonly identified, which is likely to replicated in the ECS system. We aim to generate a truly representative picture of the ECS at the protein level once sufficient sample and resources have been collected.
In summary, we have utilized genome wide gene expression to investigate a specific predefined gene set based on their previously identified involvement in the ECS. Targeted analysis of a defined gene set will reduce the burden of multiple testing in gene expression data of modest size providing the potential to uncover subtle variations. We detected differences in the gene expression across the menstrual cycle that are most pronounced during the transition from the proliferative to secretory stage and reflect a potential to dynamically modulate endocannabinoid concentrations during this period. As the activity of endocannabinoids are rapid and short-lived, we speculate that the altered regulation of expression for many components of the ECS across the menstrual cycle provides a quick acting, fine tuning potential to modulate endocannabinoid expression as needed, particularly during the transition period in which the endometrium undergoes major structural rearrangement. Of particular interest, we noted the large changes in a number of PLC enzymes, and the significant variation in FAAH and FABP5 that work in concert to deactivate endocannabinoid activity and are already receiving considerable attention as drug targets for pain, inflammation and cancer64,65. The important ability of endocannabinoid receptors to be regulated may also hold particular interest for the potential of selective targeting of patients and the personalisation of medication.
Methods
Sample collection
Samples were collected as described previously (n = 206)43 and inclusion criteria were European ancestry and within reproductive age (18–49, mean = 31.85). Exclusion criteria were the use of hormonal medication within 3 months prior to surgery, abnormalities in histopathological examination, ambiguous disease status or menstrual cycle stage. Informed consent was obtained from all subjects. A histological assessment was performed on formalin fixed paraffin embedded tissue with each sample assigned to 1 of 7 possible menstrual stages following the Noyes criteria66: menstrual (M) (n = 14), early proliferative (EP) (n = 5), mid proliferative (MP) (n = 72), late proliferative (LP) (n = 22), early secretory (ES) (n = 31), mid secretory (MS) (n = 41) and late secretory (LS) (n = 21) (Supplementary Fig. 1). If endometriosis was observed during laparoscopic surgery lesions were excised and sent to pathology for histopathological confirmation (n = 143 endometriosis cases and n = 63 non-endometriosis controls). The study was approved by the Human Research Ethics Committee (HREC) of the Royal Women’s Hospital (Projects 11-24 and 16-43 and the Melbourne IVF Project 05-11) and the University of Queensland HREC (2016000746), and all experiments were performed in accordance with relevant guidelines and regulations.
RNA extraction and sequencing
Total RNA was isolated from endometrial biopsies stored in RNAlater using the Allprep DNA/RNA Mini Kit (Qiagen, USA) as per the manufacturer’s instructions and isolated RNA samples were treated with the Turbo DNA-free kit (Thermo Fischer Scientific, USA). RNA quality was confirmed using the Agilent Bioanalyzer 2100 (Agilent Technologies, USA) with RNA integrity number (RIN) cut off values set at above 8 for inclusion and final RNA concentrations determined by the Nanodrop ND -6000 (Thermo Fisher Scientific, USA). Stranded RNA-sequencing (RNA-seq) libraries were prepared with the Illumina TruSeq Stranded Total RNA Gold protocol incorporating ribosomal depletion (Illumina, USA). The resulting libraries were pooled and sequenced with 75 bp paired-end reads on the Illumina HiSeq 4000 to a mean depth of 37,490,673 for 178 samples and with 120 bp paired-end reads on an Illumina Hi Seq 2000 (Illumina, USA) for a mean depth of 40,818,062 reads for 28 samples.
Preparation of RNA-seq data
The quality of raw RNA-seq reads were confirmed with FastQC v0.11.767 and MultiQC v1.668. Low quality reads, or reads containing HiSeq Illumina adapter sequences were trimmed using Trimmomatic v0.3669. The resulting trimmed reads were aligned to a reference assembly (Ensembl Homo sapiens GRCh38 release 91) with HISAT2 v2.0.5 and the transcript assembly performed with StringTie v1.3.170,71 and each read mapping to a known transcript counted. Transcript, exon and expression matrices in Fragments Per Kilobase of transcript per million mapped reads (FPKM) were determined with StringTie counts for each individual. Prior to normalisation of RNA-seq counts lowly expressed genes (counts per million (CPM) < 0.22) and expressed in < 90% of samples were removed. Gene counts were normalised for composition bias and total raw reads using TMM72–74 in edgeR R package v 3.22.375. The normalized counts were converted to CPM and log2 transformed (log2-CPM).
Selection of the ECS genes
A priori gene selection was performed through a literature search using the search term ‘endocannabinoid’, and combinations of ‘pathway’, ‘synthesis’, ‘metabolism’, ‘transport’ or ‘endometrium’. All genes relevant to the control of the ECS were catalogued by ENSEMBL gene expression ID and gene expression extracted from the curated database. ECS genes of interest were split into four categories based on their main function in the ECS including (i) synthesizing enzymes, (ii) receptors, (iii) transporters, and (iv) degradation enzymes. In total 70 potential genes known to play a role in the ECS were assessed (Supplementary Table 1).
Gene expression analysis
Differential gene expression was investigated both across the menstrual cycle and in women with and without endometriosis. Only genes with CPM > 0.22 in at least 90% of samples were analysed for differential expression. Seven differential gene expressions comparisons were performed across the menstrual cycle: (i) between M and EP, (ii) between EP and MP, (iii) between MP and LP, (iv) between LP and ES, (v) between ES and MS, (vi) between MS and LS and (vii) between MP and MS. Seven differential gene expressions comparisons were performed between women with and without endometriosis: (i) including stage of menstrual cycle as a covariate, (ii) within M, (iii) within MP, (iv) within LP, (v) within ES, (vi) within MS and (vii) within LS. Batch effects (flow-cell and lane) were fitted as covariates in all models. The voom function in limma76 was used to transform the normalised counts to log counts per million with associated precision weights prior to linear modelling. The pairwise comparisons described above were made using the eBayes method whereby a moderated t-statistic and log-odds of differential expression is estimated for each gene for each contrast. p values were adjusted for the number of genes tested and for the comparison of multiple cycle stage comparisons using Benjamini–Hochberg with a significance threshold of 0.05. The data underlying this article are available on the article and in its online supplementary material.
In the case of membrane receptors where positive gene expression was consistently < 90% of the samples, we explored gene expression as a binary function of expressed/not expressed and performed a logistic regression analysis as described previously43 to determine if there was as significant variation in the number of samples that show a positive expression of these genes across the menstrual cycle.
Supplementary Information
Author contributions
K.T., A.A.A., G.W.M. and B.M. were responsible for the conception and design of the present study. P.A.W.R., S.J.H., J.F.D. and W.T.T. acquired the clinical and surgical data. P.A.W.R., S.J.H., J.F.D. and W.T.T. acquired the histological and RNA data. S.M. was responsible for statistical analysis. All authors drafted the work and revised it critically for important intellectual content. All authors have read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-13488-4.
References
- 1.Tanaka K, Mayne L, Khalil A, Baartz D, Eriksson L, Mortlock SA, et al. The role of the endocannabinoid system in aetiopathogenesis of endometriosis: A potential therapeutic target. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020;244:87–94. doi: 10.1016/j.ejogrb.2019.11.012. [DOI] [PubMed] [Google Scholar]
- 2.Lu HC, Mackie K. An introduction to the endogenous cannabinoid system. Biol. Psychiatry. 2016;79(7):516–525. doi: 10.1016/j.biopsych.2015.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, et al. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372(6507):686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- 4.Wang J, Okamoto Y, Morishita J, Tsuboi K, Miyatake A, Ueda N. Functional analysis of the purified anandamide-generating phospholipase D as a member of the metallo-beta-lactamase family. J. Biol. Chem. 2006;281(18):12325–12335. doi: 10.1074/jbc.M512359200. [DOI] [PubMed] [Google Scholar]
- 5.Ueda N, Okamoto Y, Morishita J. N-Acylphosphatidylethanolamine-hydrolyzing phospholipase D: A novel enzyme of the beta-lactamase fold family releasing anandamide and other N-acylethanolamines. Life Sci. 2005;77(14):1750–1758. doi: 10.1016/j.lfs.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 6.Okamoto Y, Morishita J, Tsuboi K, Tonai T, Ueda N. Molecular characterization of a phospholipase D generating anandamide and its congeners. J. Biol. Chem. 2004;279(7):5298–5305. doi: 10.1074/jbc.M306642200. [DOI] [PubMed] [Google Scholar]
- 7.Liu J, Wang L, Harvey-White J, Osei-Hyiaman D, Razdan R, Gong Q, et al. A biosynthetic pathway for anandamide. Proc. Natl. Acad. Sci. U. S. A. 2006;103(36):13345–13350. doi: 10.1073/pnas.0601832103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu J, Wang L, Harvey-White J, Huang BX, Kim HY, Luquet S, et al. Multiple pathways involved in the biosynthesis of anandamide. Neuropharmacology. 2008;54(1):1–7. doi: 10.1016/j.neuropharm.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun YX, Tsuboi K, Okamoto Y, Tonai T, Murakami M, Kudo I, et al. Biosynthesis of anandamide and N-palmitoylethanolamine by sequential actions of phospholipase A2 and lysophospholipase D. Biochem. J. 2004;380(Pt 3):749–756. doi: 10.1042/bj20040031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsuboi K, Ikematsu N, Uyama T, Deutsch DG, Tokumura A, Ueda N. Biosynthetic pathways of bioactive N-acylethanolamines in brain. CNS Neurol. Disord. Drug Targets. 2013;12(1):7–16. doi: 10.2174/1871527311312010005. [DOI] [PubMed] [Google Scholar]
- 11.Simon GM, Cravatt BF. Endocannabinoid biosynthesis proceeding through glycerophospho-N-acyl ethanolamine and a role for alpha/beta-hydrolase 4 in this pathway. J. Biol. Chem. 2006;281(36):26465–26472. doi: 10.1074/jbc.M604660200. [DOI] [PubMed] [Google Scholar]
- 12.Simon GM, Cravatt BF. Characterization of mice lacking candidate N-acyl ethanolamine biosynthetic enzymes provides evidence for multiple pathways that contribute to endocannabinoid production in vivo. Mol. Biosyst. 2010;6(8):1411–1418. doi: 10.1039/c000237b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384(6604):83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- 14.Giang DK, Cravatt BF. Molecular characterization of human and mouse fatty acid amide hydrolases. Proc. Natl. Acad. Sci. U. S. A. 1997;94(6):2238–2242. doi: 10.1073/pnas.94.6.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rouzer CA, Marnett LJ. Endocannabinoid oxygenation by cyclooxygenases, lipoxygenases, and cytochromes P450: Cross-talk between the eicosanoid and endocannabinoid signaling pathways. Chem. Rev. 2011;111(10):5899–5921. doi: 10.1021/cr2002799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kozak KR, Crews BC, Morrow JD, Wang LH, Ma YH, Weinander R, et al. Metabolism of the endocannabinoids, 2-arachidonylglycerol and anandamide, into prostaglandin, thromboxane, and prostacyclin glycerol esters and ethanolamides. J. Biol. Chem. 2002;277(47):44877–44885. doi: 10.1074/jbc.M206788200. [DOI] [PubMed] [Google Scholar]
- 17.Hampson AJ, Hill WA, Zan-Phillips M, Makriyannis A, Leung E, Eglen RM, et al. Anandamide hydroxylation by brain lipoxygenase: Metabolite structures and potencies at the cannabinoid receptor. Biochim. Biophys. Acta. 1995;1259(2):173–179. doi: 10.1016/0005-2760(95)00157-8. [DOI] [PubMed] [Google Scholar]
- 18.Van Der Stelt M, Noordermeer MA, Kiss T, Van Zadelhoff G, Merghart B, Veldink GA, et al. Formation of a new class of oxylipins from N-acyl(ethanol)amines by the lipoxygenase pathway. Eur. J. Biochem. 2000;267(7):2000–2007. doi: 10.1046/j.1432-1327.2000.01203.x. [DOI] [PubMed] [Google Scholar]
- 19.Snider NT, Walker VJ, Hollenberg PF. Oxidation of the endogenous cannabinoid arachidonoyl ethanolamide by the cytochrome P450 monooxygenases: Physiological and pharmacological implications. Pharmacol. Rev. 2010;62(1):136–154. doi: 10.1124/pr.109.001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Urquhart P, Nicolaou A, Woodward DF. Endocannabinoids and their oxygenation by cyclo-oxygenases, lipoxygenases and other oxygenases. Biochim. Biophys. Acta. 2015;1851(4):366–376. doi: 10.1016/j.bbalip.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 21.Di Marzo V, Bisogno T, De Petrocellis L, Melck D, Orlando P, Wagner JA, et al. Biosynthesis and inactivation of the endocannabinoid 2-arachidonoylglycerol in circulating and tumoral macrophages. Eur. J. Biochem. 1999;264(1):258–267. doi: 10.1046/j.1432-1327.1999.00631.x. [DOI] [PubMed] [Google Scholar]
- 22.Sugiura T, Kobayashi Y, Oka S, Waku K. Biosynthesis and degradation of anandamide and 2-arachidonoylglycerol and their possible physiological significance. Prostaglandins Leukot Essent. Fatty Acids. 2002;66(2–3):173–192. doi: 10.1054/plef.2001.0356. [DOI] [PubMed] [Google Scholar]
- 23.Higgs HN, Glomset JA. Identification of a phosphatidic acid-preferring phospholipase A1 from bovine brain and testis. Proc. Natl. Acad. Sci. U. S. A. 1994;91(20):9574–9578. doi: 10.1073/pnas.91.20.9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Marzo V, De Petrocellis L, Bisogno T, Melck D. Metabolism of anandamide and 2-arachidonoylglycerol: An historical overview and some recent developments. Lipids. 1999;34(Suppl):S319–S325. doi: 10.1007/BF02562332. [DOI] [PubMed] [Google Scholar]
- 25.Blankman JL, Simon GM, Cravatt BF. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem. Biol. 2007;14(12):1347–1356. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sang N, Zhang J, Chen C. COX-2 oxidative metabolite of endocannabinoid 2-AG enhances excitatory glutamatergic synaptic transmission and induces neurotoxicity. J. Neurochem. 2007;102(6):1966–1977. doi: 10.1111/j.1471-4159.2007.04668.x. [DOI] [PubMed] [Google Scholar]
- 27.Gentilini D, Besana A, Vigano P, Dalino P, Vignali M, Melandri M, et al. Endocannabinoid system regulates migration of endometrial stromal cells via cannabinoid receptor 1 through the activation of PI3K and ERK1/2 pathways. Fertil. Steril. 2010;93(8):2588–2593. doi: 10.1016/j.fertnstert.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 28.McHugh D, Page J, Dunn E, Bradshaw HB. Delta(9)-Tetrahydrocannabinol and N-arachidonyl glycine are full agonists at GPR18 receptors and induce migration in human endometrial HEC-1B cells. Br. J. Pharmacol. 2012;165(8):2414–2424. doi: 10.1111/j.1476-5381.2011.01497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leconte M, Nicco C, Ngo C, Arkwright S, Chereau C, Guibourdenche J, et al. Antiproliferative effects of cannabinoid agonists on deep infiltrating endometriosis. Am. J. Pathol. 2010;177(6):2963–2970. doi: 10.2353/ajpath.2010.100375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bilgic E, Guzel E, Kose S, Aydin MC, Karaismailoglu E, Akar I, et al. Endocannabinoids modulate apoptosis in endometriosis and adenomyosis. Acta Histochem. 2017;119(5):523–532. doi: 10.1016/j.acthis.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 31.Resuehr D, Glore DR, Taylor HS, Bruner-Tran KL, Osteen KG. Progesterone-dependent regulation of endometrial cannabinoid receptor type 1 (CB1-R) expression is disrupted in women with endometriosis and in isolated stromal cells exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) Fertil. Steril. 2012;98(4):948–56.e1. doi: 10.1016/j.fertnstert.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iuvone T, De Filippis D, Di Spiezio SA, D'Amico A, Simonetti S, Sparice S, et al. Selective CB2 up-regulation in women affected by endometrial inflammation. J. Cell. Mol. Med. 2008;12(2):661–670. doi: 10.1111/j.1582-4934.2007.00085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galve-Roperh I, Chiurchiù V, Díaz-Alonso J, Bari M, Guzmán M, Maccarrone M. Cannabinoid receptor signaling in progenitor/stem cell proliferation and differentiation. Prog. Lipid Res. 2013;52(4):633–650. doi: 10.1016/j.plipres.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 34.Critchley HOD, Maybin JA, Armstrong GM, Williams ARW. Physiology of the endometrium and regulation of menstruation. Physiol. Rev. 2020;100(3):1149–1179. doi: 10.1152/physrev.00031.2019. [DOI] [PubMed] [Google Scholar]
- 35.Gargett CE, Schwab KE, Deane JA. Endometrial stem/progenitor cells: The first 10 years. Hum. Reprod. Update. 2016;22(2):137–163. doi: 10.1093/humupd/dmv051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cousins FL, Pandoy R, Jin S, Gargett CE. The elusive endometrial epithelial stem/progenitor cells. Front. Cell Dev. Biol. 2021;9:640319. doi: 10.3389/fcell.2021.640319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Almada M, Amaral C, Diniz-da-Costa M, Correia-da-Silva G, Teixeira NA, Fonseca BM. The endocannabinoid anandamide impairs in vitro decidualization of human cells. Reproduction. 2016;152(4):351–361. doi: 10.1530/REP-16-0364. [DOI] [PubMed] [Google Scholar]
- 38.Moghadam KK, Kessler CA, Schroeder JK, Buckley AR, Brar AK, Handwerger S. Cannabinoid receptor I activation markedly inhibits human decidualization. Mol. Cell Endocrinol. 2005;229(1–2):65–74. doi: 10.1016/j.mce.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 39.Maia J, Fonseca BM, Teixeira N, Correia-da-Silva G. The fundamental role of the endocannabinoid system in endometrium and placenta: Implications in pathophysiological aspects of uterine and pregnancy disorders. Hum. Reprod. Update. 2020;26(4):586–602. doi: 10.1093/humupd/dmaa005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stasiulewicz A, Znajdek K, Grudzien M, Pawinski T, Sulkowska AJI. A guide to targeting the endocannabinoid system in drug design. Int. J. Mol. Sci. 2020;21(8):2778. doi: 10.3390/ijms21082778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447):1789–1799. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- 42.Rowlands IJ, Abbott JA, Montgomery GW, Hockey R, Rogers P, Mishra GD. Prevalence and incidence of endometriosis in Australian women: A data linkage cohort study. BJOG. 2021;128(4):657–665. doi: 10.1111/1471-0528.16447. [DOI] [PubMed] [Google Scholar]
- 43.Fung JN, Mortlock S, Girling JE, Holdsworth-Carson SJ, Teh WT, Zhu Z, et al. Genetic regulation of disease risk and endometrial gene expression highlights potential target genes for endometriosis and polycystic ovarian syndrome. Sci. Rep. 2018;8(1):11424. doi: 10.1038/s41598-018-29462-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mortlock S, Kendarsari RI, Fung JN, Gibson G, Yang F, Restuadi R, et al. Tissue specific regulation of transcription in endometrium and association with disease. Hum. Reprod. 2020;35(2):377–393. doi: 10.1093/humrep/dez279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guindon J, Hohmann AG. The endocannabinoid system and pain. CNS Neurol. Disord. Drug Targets. 2009;8(6):403–421. doi: 10.2174/187152709789824660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Di Blasio AM, Vignali M, Gentilini D. The endocannabinoid pathway and the female reproductive organs. J. Mol. Endocrinol. 2013;50(1):R1–9. doi: 10.1530/JME-12-0182. [DOI] [PubMed] [Google Scholar]
- 47.Karasu T, Marczylo TH, Maccarrone M, Konje JC. The role of sex steroid hormones, cytokines and the endocannabinoid system in female fertility. Hum. Reprod. Update. 2011;17(3):347–361. doi: 10.1093/humupd/dmq058. [DOI] [PubMed] [Google Scholar]
- 48.Maccarrone M, Bari M, Di Rienzo M, Finazzi-Agro A, Rossi A. Progesterone activates fatty acid amide hydrolase (FAAH) promoter in human T lymphocytes through the transcription factor Ikaros. Evidence for a synergistic effect of leptin. J. Biol. Chem. 2003;278(35):32726–32732. doi: 10.1074/jbc.M302123200. [DOI] [PubMed] [Google Scholar]
- 49.Guo Y, Wang H, Okamoto Y, Ueda N, Kingsley PJ, Marnett LJ, et al. N-Acylphosphatidylethanolamine-hydrolyzing phospholipase D is an important determinant of uterine anandamide levels during implantation. J. Biol. Chem. 2005;280(25):23429–23432. doi: 10.1074/jbc.C500168200. [DOI] [PubMed] [Google Scholar]
- 50.El-Talatini MR, Taylor AH, Konje JC. The relationship between plasma levels of the endocannabinoid, anandamide, sex steroids, and gonadotrophins during the menstrual cycle. Fertil. Steril. 2010;93(6):1989–1996. doi: 10.1016/j.fertnstert.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 51.Taylor AH, Abbas MS, Habiba MA, Konje JC. Histomorphometric evaluation of cannabinoid receptor and anandamide modulating enzyme expression in the human endometrium through the menstrual cycle. Histochem. Cell Biol. 2010;133(5):557–565. doi: 10.1007/s00418-010-0695-9. [DOI] [PubMed] [Google Scholar]
- 52.Rajala RVS, Anderson RE. Focus on molecules: Phosphatidylinositol-4,5-bisphosphate (PIP2) Exp. Eye Res. 2010;91(3):324–325. doi: 10.1016/j.exer.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murataeva N, Straiker A, Mackie K. Parsing the players: 2-arachidonoylglycerol synthesis and degradation in the CNS. Br. J. Pharmacol. 2014;171(6):1379–1391. doi: 10.1111/bph.12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maccarrone M, Valensise H, Bari M, Lazzarin N, Romanini C, Finazzi-Agro A. Progesterone up-regulates anandamide hydrolase in human lymphocytes: Role of cytokines and implications for fertility. J. Immunol. 2001;166(12):7183–7189. doi: 10.4049/jimmunol.166.12.7183. [DOI] [PubMed] [Google Scholar]
- 55.Gasperi V, Fezza F, Spagnuolo P, Pasquariello N, Maccarrone M. Further insights into the regulation of human FAAH by progesterone and leptin implications for endogenous levels of anandamide and apoptosis of immune and neuronal cells. Neurotoxicology. 2005;26(5):811–817. doi: 10.1016/j.neuro.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 56.Pavone ME, Reierstad S, Sun H, Milad M, Bulun SE, Cheng YH. Altered retinoid uptake and action contributes to cell survival in endometriosis. J. Clin. Endocrinol. Metab. 2010;95(11):E300–E309. doi: 10.1210/jc.2010-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peng X, Studholme K, Kanjiya MP, Luk J, Bogdan D, Elmes MW, et al. Fatty-acid-binding protein inhibition produces analgesic effects through peripheral and central mechanisms. Mol. Pain. 2017;13:1744806917697007. doi: 10.1177/1744806917697007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maccarrone M, Di Rienzo M, Finazzi-Agro A, Rossi A. Leptin activates the anandamide hydrolase promoter in human T lymphocytes through STAT3. J. Biol. Chem. 2003;278(15):13318–13324. doi: 10.1074/jbc.M211248200. [DOI] [PubMed] [Google Scholar]
- 59.MacCarrone M, De Felici M, Bari M, Klinger F, Siracusa G, Finazzi-Agro A. Down-regulation of anandamide hydrolase in mouse uterus by sex hormones. Eur. J. Biochem. 2000;267(10):2991–2997. doi: 10.1046/j.1432-1033.2000.01316.x. [DOI] [PubMed] [Google Scholar]
- 60.Lazzarin N, Valensise H, Bari M, Ubaldi F, Battista N, Finazzi-Agro A, et al. Fluctuations of fatty acid amide hydrolase and anandamide levels during the human ovulatory cycle. Gynecol. Endocrinol. 2004;18(4):212–218. doi: 10.1080/09513590410001692492. [DOI] [PubMed] [Google Scholar]
- 61.Kaczocha M, Glaser ST, Deutsch DG. Identification of intracellular carriers for the endocannabinoid anandamide. Proc. Natl. Acad. Sci. U. S. A. 2009;106(15):6375–6380. doi: 10.1073/pnas.0901515106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hall OJ, Klein SL. Progesterone-based compounds affect immune responses and susceptibility to infections at diverse mucosal sites. Mucosal. Immunol. 2017;10(5):1097–1107. doi: 10.1038/mi.2017.35. [DOI] [PubMed] [Google Scholar]
- 63.Sanchez AM, Cioffi R, Vigano P, Candiani M, Verde R, Piscitelli F, et al. Elevated systemic levels of endocannabinoids and related mediators across the menstrual cycle in women with endometriosis. Reprod. Sci. 2016;23(8):1071–1079. doi: 10.1177/1933719116630414. [DOI] [PubMed] [Google Scholar]
- 64.Schlosburg JE, Kinsey SG, Lichtman AH. Targeting fatty acid amide hydrolase (FAAH) to treat pain and inflammation. AAPS J. 2009;11(1):39–44. doi: 10.1208/s12248-008-9075-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brunetti L, Loiodice F, Piemontese L, Tortorella P, Laghezza A. New approaches to cancer therapy: Combining fatty acid amide hydrolase (FAAH) inhibition with peroxisome proliferator-activated receptors (PPARs) activation. J. Med. Chem. 2019;62(24):10995–11003. doi: 10.1021/acs.jmedchem.9b00885. [DOI] [PubMed] [Google Scholar]
- 66.Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Fertil. Steril. 1950;1(1):3–25. doi: 10.1016/S0015-0282(16)30062-0. [DOI] [PubMed] [Google Scholar]
- 67.Andrews. FastQC: A quality control tool for high throughput sequence data. Available online at http://www.bioinformatics.babraham.ac.uk/projects/fastqc (2010).
- 68.Ewels P, Magnusson M, Lundin S, Käller M. MultiQC: Summarize analysis results for multiple tools and samples in a single report. Bioinformatics. 2016;32(19):3047–3048. doi: 10.1093/bioinformatics/btw354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bolger AM, Lohse M, Usadel B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pertea M, Kim D, Pertea G, Leek J, Salzberg S. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016;11(9):1650–1667. doi: 10.1038/nprot.2016.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pertea M, Pertea G, Antonescu C, Chang T-C, Mendell J, Salzberg S. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015;33(3):290–295. doi: 10.1038/nbt.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fadista J, Vikman P, Laakso EO, Mollet IG, Esguerra JL, Taneera J, et al. Global genomic and transcriptomic analysis of human pancreatic islets reveals novel genes influencing glucose metabolism. Proc. Natl. Acad. Sci. 2014;111(38):13924–13929. doi: 10.1073/pnas.1402665111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Seo M, Kim K, Yoon J, Jeong JY, Lee H-J, Cho S, et al. RNA-seq analysis for detecting quantitative trait-associated genes. Sci. Rep. 2016;6:24375. doi: 10.1038/srep24375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Taneera J, Fadista J, Ahlqvist E, Atac D, Ottosson-Laakso E, Wollheim CB, et al. Identification of novel genes for glucose metabolism based upon expression pattern in human islets and effect on insulin secretion and glycemia. Hum. Mol. Genet. 2015;24(7):1945–1955. doi: 10.1093/hmg/ddu610. [DOI] [PubMed] [Google Scholar]
- 75.Robinson MD, McCarthy DJ, Smyth GK. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26(1):139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Law CW, Chen Y, Shi W, Smyth GK. voom: Precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 2014;15(2):R29. doi: 10.1186/gb-2014-15-2-r29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.