Abstract
Inflammatory myofibroblastic tumour (IMT) is an unusual myofibroblastic spindle cell neoplasm that is rarely discovered in the airway of adults. Previously, it was regarded as a reactive lesion and was infamously known as inflammatory pseudotumour before recent insights revealed that significant majority of cases harboured neoplastic genes. Diagnosis is difficult as clinical presentation and imaging findings are non‐specific. Diagnosis and a favourable prognosis require the complete resection of the tumour. Detection of the anaplastic lymphoma kinase expression via immunohistochemistry expedites diagnosis. We report a young adult with an endotracheal mass occluding the central airway. The patient successfully underwent bronchoscopic resection using interventional techniques. IMT was diagnosed. No recurrence was seen after a year of surveillance. Due to the rarity of the disease, the recurrence rates for large airway disease is unknown. Recurrence rates for pulmonary lesions is reported to be lower compared to extrapulmonary IMTs and recurrence is unlikely if compete surgical excision is achieved.
Keywords: anaplastic lymphoma kinase, central airway, endotracheal, inflammatory myofibroblastic tumour
We report a case of endotracheal inflammatory myofibroblastic tumour resected using diathermy snaring and debulking via rigid bronchoscopy in a young adult.
INTRODUCTION
Inflammatory myofibroblastic tumour (IMT) is an uncommon mesenchymal neoplasm characterized by myofibroblastic spindle cell proliferation with a variable non‐specific inflammatory cell infiltration mainly consisting of lymphocytes, plasma cells and eosinophils. Pulmonary IMT was first reported in 1939 and constitutes less than 1% of all adult respiratory tract neoplasms. 1 , 2 Although it may commonly occur in the lungs of children and adolescents, pulmonary and endotracheal IMTs are extremely rare in adults.
In the past, IMT was regarded as a benign reactive lesion and a subset of inflammatory pseudotumour with interchangeable diverse nomenclatures which include plasma cell granuloma, xanthogranuloma and fibrous histiocytoma. 3 , 4 Although the exact aetiology and pathophysiology of IMT remain unclear, recent insights revealed that 50%–70% of IMTs harbour genes and chromosomal abnormalities which encode mitogen‐activated protein kinase pathways. 5
The diagnosis and prognosis are heavily reliant on the achievement of complete surgical excision. Histopathological diagnosis is often very challenging and requires excision of the tumour. Complete surgical resection when possible ensures good survival and low recurrence rates. 6 Bronchoscopic techniques are minimally invasive and have the advantage to resect lesions localized in the airways without compromising lung function. 7 We report a case of endotracheal IMT resected using diathermy snaring and debulking via rigid bronchoscopy in a young adult.
CASE REPORT
A 26‐year‐old man with no comorbidities presented with haemoptysis and dysphagia for 2 years. His haemoptysis was mild, not exceeding two tablespoons of fresh blood occurring once or twice in a month. He also experienced dysphagia to solid food which worsened 2 months prior to presentation. He had a history of smoking (1 pack per day for the past 10 years). Otherwise, physical examination, baseline laboratory investigations and chest radiograph findings were all unremarkable.
Contrast computed tomography (CT) of the chest was abnormal and demonstrated a well‐defined endotracheal round mass with smooth margins measuring 1.6 cm in length. The mass was 4.5 cm from the main carina and the tracheal lumen was narrowed more than 50% with the narrowest diameter of 0.5 cm. There were no other significant lung masses or nodules, pleural effusion or lymphadenopathy reported (Figure 1).
FIGURE 1.
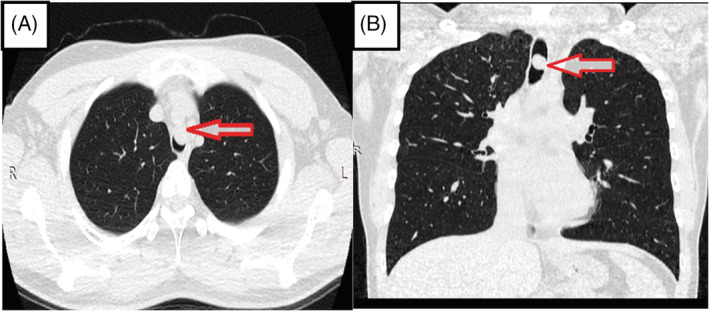
Computed tomography of the chest (A, axial view; B, coronal view) demonstrated a well‐defined endotracheal round mass with smooth margins measuring 1.6 cm in length.
Urgent rigid bronchoscopy revealed a polypoidal mass occluding 90% of the tracheal lumen (Figure 2A). The tumour was completely resected using a diathermy snare and the tumour base was debulked and treated with diathermy. Haemostasis was secured with endotracheal instillation of adrenaline (Figure 2B).
FIGURE 2.
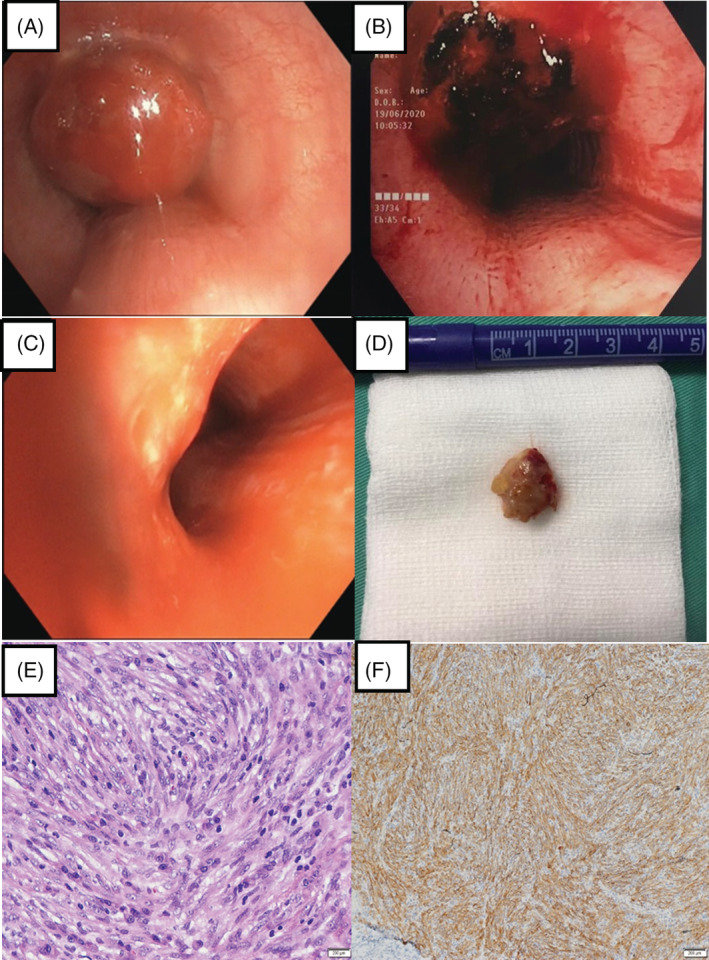
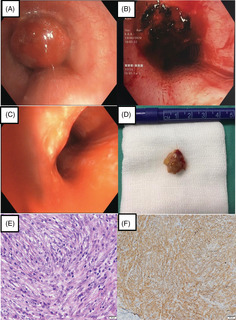
Bronchoscopic view of the endotracheal mass (A) prior to intervention, (B) post diathermy snaring and debulking and (C) surveillance 1 year later. Pathology findings: (D) gross appearance of the resected mass. Histopathology findings: (E) presence of spindle cell tumour cells with prominent fusiform nuclei and eosinophilic cytoplasm arranged in fascicles (haematoxylin and eosin stain, original magnification 40×). Immunohistochemistry staining: (F) anaplastic lymphoma kinase positive with cytoplasmic pattern (original magnification 100×)
On gross pathology, a 2‐cm polypoidal mass with greyish surface was retrieved through bronchoscopic resection (Figure 2D). Subsequent histological evaluation revealed spindle cell tumour cells with prominent fusiform or oval nuclei and eosinophilic cytoplasm arranged in fascicles and vague storiform pattern. The stroma had an intense inflammatory cell infiltration especially by plasma cells and lymphocytes (Figure 2E). On immunohistochemistry, tumour cells stained positively for smooth muscle actin with a tram‐track pattern and a cytoplasmic pattern for anaplastic lymphoma kinase (ALK) (Figure 2F) as well as negatively for cytokeratin stains, desmin for smooth muscle cells, CD 34 and S‐100 ruling out a granular tumour. IMT was diagnosed.
Surveillance flexible bronchoscopy after 6 months showed a well‐healed tumour base. Serial bronchoscopy (Figure 2C) and CT scans showed no recurrence in 12 months.
DISCUSSION
IMT is a rare soft tissue neoplasm of mesenchymal origin that may arise in a wide variety of sites such as the abdomen, mesentery, lung, head, neck, extremities, genito‐urinary tract and orbit. However, it commonly occurs in the lungs of children and adolescents. Previously, IMT was perceived as a benign reactive lesion and was largely known as inflammatory pseudotumour. Airway IMTs are extremely rare in adults with a prevalence of not more than 12% among pulmonary IMTs. 8 The data on the epidemiology of airway IMTs are limited due to the low prevalence of the disease in the adult population. To the best of our knowledge, only 11 cases of adult tracheal IMTs have been reported, although the prevalence is speculated to be higher due to potential unreported cases (Table 1). 7 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 The disease is mostly seen before the age of 50, but has also been described as late as in the eight decades of life. There was also no gender predominance or ethnic and geographic predispositions for the development of the disease. 19
TABLE 1.
Reported adult endotracheal inflammatory myofibroblastic tumour
| Year | First author | Age | Presenting symptom | Histological diagnosis | Treatment | Outcome |
|---|---|---|---|---|---|---|
| 1991 | Satomi 10 | 55 | Stridor | Plasma cell granuloma | Bronchoscopic resection with laser | Not disclosed |
| 1993 | Ishii 11 | 61 | Dyspnoea and wheezing | IMT | Surgery | Not disclosed |
| 2002 | Amir 12 | 21 | Dyspnoea and stridor | IMT | Surgery | Not disclosed |
| 2003 | Restrepo 13 | 20 | Dyspnoea and cough | IMT | Surgery | Not disclosed |
| 2004 | Nikanne 14 | 21 | Dyspnoea and cough | IMT | Bronchoscopic resection | Not disclosed |
| 2006 | Belák 15 | 45 | Dyspnoea and stridor | IMT | Surgery | Not disclosed |
| 2006 | Ono 16 | 45 | Dyspnoea | IMT | Bronchoscopic resection with laser | Not disclosed |
| 2009 | He 17 | 28 | Dyspnoea | IMT | Surgery | Not disclosed |
| 2009 | Fabre 9 | 19 | Not available | IMT | Surgery | Not disclosed |
| 2010 | Andrade 7 | 31 | Cough and dyspnoea | IMT | Bronchoscopic resection and lobectomy | No recurrence after 6 years |
| 2011 | Koch 18 | 57 | Cough, haemoptysis | IMT | Bronchoscopic resection | No recurrence after 6 months |
Abbreviation: IMT, inflammatory myofibroblastic tumour.
The clinical and radiological diagnoses of IMTs are often difficult due to the non‐specific presentation. Although the majority of patients have asymptomatic disease that are found incidentally on imaging, patients with tracheobronchial IMTs are often recognized earlier as they are likely to present with symptoms of airway obstruction, respiratory distress and lung collapse such as dyspnoea, cough and haemoptysis. 20 On imaging, pulmonary IMTs may be detected as peripheral lung nodules and masses as well as hilar and mediastinal lymphadenopathies. 21 Our patient presented 2 years from onset of symptoms due to the lack of obstructive respiratory symptoms. The dysphagia which was experienced by our patient was an atypical symptom and was attributed to the compression of the oesophagus as it resolved after the surgical excision of the endotracheal mass.
The ALK gene locus on 2p23 encodes a classical receptor tyrosine kinase that was detected in anaplastic large‐cell lymphoma, diffuse large‐cell lymphoma and non‐small cell lung cancer, and was also discovered in 50% of IMTs. 22 , 23 This revelation gives us the understanding that IMTs are a true neoplastic process and they were classified by the World Health Organization as tumours of intermediate biological potential due to a tendency for local recurrence and the small risk for distant metastasis. 24 ALK expression can be detected by immunohistochemistry as well as fluorescence in situ hybridization, and is found to be more common in younger patients. 25 Despite ALK‐positive IMT has a tendency for recurrence, it carries a favourable prognosis as ALK negativity is associated with metastasis. 26 Our patient manifested ALK expression which was detected by immunohistochemistry.
Diagnosis of IMT is very difficult and often only possible after complete surgical excision of the lesion. Sampling from bronchoscopy or needle biopsy may yield small specimens that are insufficient for diagnosis and it was found that only 6.3% of IMT cases are diagnosed based on preoperative sampling methods. 20 These methods may be convenient but are unreliable as the pathologist may need to evaluate the complete lesion to make a confident diagnosis of IMT. In the past, patients with IMT commonly underwent multiple biopsy procedures to establish a diagnosis. Classical histological features of IMT include the presence of cellular spindle cell proliferation in a myxoid to collagenous stroma with a prominent inflammatory infiltrate composed primarily of plasma cells and lymphocytes, with occasional eosinophils and neutrophils. Due to the predominance of inflammatory cells, immunohistochemistry is necessary to distinguish IMT from other lesions with similar histological features. Comprehensive histopathological and immunohistochemical evaluation are required to avoid misdiagnosis. Complete surgical excision of the endotracheal mass and the presence of classical histological features seen in our patient expedited diagnosis.
Radical bronchoscopic resection via rigid bronchoscopy has been the mainstay of treatment of airway IMTs to prevent recurrence. 7 Incomplete resections of pulmonary masses may pose a 60% recurrence rate as described in recent studies. 27 Recurrences have been reported among patients with tumours in the larynx where complete surgical resection is challenging. 28 Complete surgical excision of a solitary lesion is necessary to ensure a favourable prognosis and improved survival. Evidence for efficacious treatment alternative to surgery remains limited. We performed diathermy snaring and debulking of the endotracheal mass via rigid bronchoscopy.
The recurrence rates specifically for tracheal IMTs are unknown, but for pulmonary IMTs it was reported to be low around 2%. Although it is remarkably lower than the recurrence rates for extrapulmonary IMTs, reported as 25% in previous studies, regular imaging and bronchoscopy to detect recurrence are recommended within the first 2 years after diagnosis. 29 Distant metastases is also rare, occurring in 5%–11% of cases especially in children and extrapulmonary IMTs. Common sites include the lung, brain, liver and bone. 30 Due to the rarity of the condition, predictors of disease behaviour remain understudied. Overall prognosis is excellent with a 10‐year survival of 89% and is dependent on the size of primary lesion and whether complete surgical resection is feasible. 9 Our patient remained asymptomatic following 1 year of surveillance without any evidence of recurrence.
In conclusion, tracheal IMT is a rare cause of central airway occlusion in adults. Prompt recognition will require early imaging and bronchoscopy. Endoluminal lesions should be resected completely using bronchoscopic techniques to expedite diagnosis. In most cases, surgical resection alone is sufficient to deter recurrence and ensure a favourable prognosis. Surveillance imaging and bronchoscopy are recommended for the subsequent 2 years to anticipate recurrence.
AUTHOR CONTRIBUTION
Albert Iruthiaraj Lourdesamy Anthony drafted the manuscript. All authors were involved in the care of the patient. All authors critically revised the draft manuscript and approved the final manuscript.
CONFLICT OF INTEREST
None declared.
ETHICS STATEMENT
The authors declare that appropriate written informed consent was obtained for the publication of this manuscript and accompanying images.
ACKNOWLEDGMENTS
The authors would like to thank the Director General of Health Malaysia for permission to publish this paper. The authors also thank Dr. Noraini Mohd Dusa (Department of Pathology, Hospital Kuala Lumpur) for her contributions in the histopathological analysis.
Lourdesamy Anthony AI, Satnam Singh TK, Ng KL, Abdul Rahaman JA. Endotracheal inflammatory myofibroblastic tumour: A rare cause of central airway occlusion in adults. Respirology Case Reports. 2022;10:e0984. 10.1002/rcr2.984
Associate Editor: James C. M. Ho
DATA AVAILABILITY STATEMENT
Data are available on request from the authors.
REFERENCES
- 1. Brunn H. Two interesting benign lung tumors of contradictory histopathology. J Thorac Surg. 1939;9:119–31. [Google Scholar]
- 2. Sakurai H, Hasegawa T, Watanabe S, Suzuki K, Asamura H, Tsuchiya R. Inflammatory myofibroblastic tumor of the lung. Eur J Cardiothorac Surg. 2004;25(2):155–9. [DOI] [PubMed] [Google Scholar]
- 3. Berardi RS, Lee SS, Chen HP, Stines GJ. Inflammatory pseudotumors of the lung. Surg Gynecol Obstet. 1983;156:89–96. [PubMed] [Google Scholar]
- 4. Grossman RE, Bemis EL, Pemberton AH, Narodick BG. Fibrous histiocytoma or xanthoma of the lung with bronchial involvement. J Thorac Cardiovasc Surg. 1973;65:653–7. [PubMed] [Google Scholar]
- 5. Antonescu CR, Albert JH, Zhang L. Molecular characterization of inflammatory myofibroblastic tumors with frequent ALK and ROS1 gene fusions and rare novel RET rearrangement. Am J Surg Pathol. 2015;39:957–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen CK, Jan CI, Tsai JS. Inflammatory myofibroblastic tumor of the lung – a case report. J Cardiothorac Surg. 2010;5:55. 10.1186/1749-8090-5-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Andrade FM, Abou‐Mourad OM, Judice LF, Carvalho‐Filho ABCB, Schau B, Carvalho ACG. Endotracheal inflammatory pseudotumor: the role of interventional bronchoscopy. Ann Thorac Surg. 2010;90(3):e36–7. 10.1016/j.athoracsur.2010.06.013 [DOI] [PubMed] [Google Scholar]
- 8. Calderazzo M, Gallelli A, Barbieri V, Roccia F, Pelaia G, Tranfa CME, et al. Inflammatory pseudotumour of the lung presenting as an airway obstructive syndrome. Respir Med. 1997;91:381–4. [DOI] [PubMed] [Google Scholar]
- 9. Fabre D, Fadel E, Singhal S, de Montpreville V, Mussot S, Mercier O. Complete resection of pulmonary inflammatory pseudotumors has excellent long‐term prognosis. J Thorac Cardiovasc Surg. 2009;137(2):435–40. [DOI] [PubMed] [Google Scholar]
- 10. Satomi F, Mori H, Ogasawara H, Kumoi T, Uematsu K. Subglottic plasma cell granuloma: report of a case. Auris Nasus Larynx. 1991;18(4):391–9. [DOI] [PubMed] [Google Scholar]
- 11. Ishii Y, Inoue F, Kamikawa Y, Shin R, Orita K, Seo K. A case report of tracheal inflammatory pseudotumor. Nihon Kyobu Geka Gakkai Zasshi. 1993;41(4):672–7. (in Japanese). [PubMed] [Google Scholar]
- 12. Amir R, Danahey D, Ferrer K, Maffee M. Inflammatory myofibroblastic tumor presenting with tracheal obstruction in a pregnant woman. Am J Otolaryngol. 2002;23(6):362–7. [DOI] [PubMed] [Google Scholar]
- 13. Restrepo S, Mastrogiovanni LP, Palacios E. Inflammatory pseudotumor of the trachea. Ear Nose Throat J. 2003;82(7):510–2. [PubMed] [Google Scholar]
- 14. Nikanne E, Sopanen J, Seppa A. Inflammatory pseudotumor of the trachea. Otolaryngol Head Neck Surg. 2004;130(2):274–6. [DOI] [PubMed] [Google Scholar]
- 15. Belák J, Janík M, Kudlác M, Cavarga I, Michlík J, Kmecová L. Tracheal tumor: a case review. Rozhl Chir. 2006;85(5):220–2. (in Slovak). [PubMed] [Google Scholar]
- 16. Ono Y, Miyoshi T, Inutsuka K, Shiraishi T, Nabeshima K, Shirakusa T. Inflammatory myofibroblastic tumor of the trachea; report of a case. Kyobu Geka. 2006;59(9):871–5. (in Japanese). [PubMed] [Google Scholar]
- 17. He J, Xu X, Chen M, Li S, Yin W, Wang S. Novel method to repair tracheal defect by pectoralis major myocutaneous flap. Ann Thorac Surg. 2009;88(1):288–91. [DOI] [PubMed] [Google Scholar]
- 18. Koch JA, Dorn P, Rausch T, Ris HB, Lehr HA, Schafer SC. Inflammatory myofibroblastic tumor of the trachea with concomitant granulomatous lymph node lesions. Case Rep Med. 2011;2011:151729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pettinato G, Manivel JC, De Rose N, Dehner LP. Inflammatory myofibroblastic tumor (plasma cell granuloma): clinicopathologic study of 20 cases with immunohistochemical and ultrastructural observations. Am J Clin Pathol. 1990;94:538–46. [DOI] [PubMed] [Google Scholar]
- 20. Lee HJ, Kim JS, Choi YS, Kim K, Shim YM, Han J, et al. Treatment of inflammatory myofibroblastic tumor of the chest: the extent of resection. Ann Thorac Surg. 2007;84:221–4. [DOI] [PubMed] [Google Scholar]
- 21. Agrons GA, Rosado‐de‐Christenson ML, Kirejczyk WM, Conran RM, Stocker JT. Pulmonary inflammatory pseudotumor: radiologic features. Radiology. 1998;206(2):511–8. 10.1148/radiology.206.2.9457206 [DOI] [PubMed] [Google Scholar]
- 22. Hallberg B, Palmer RH. Mechanistic insight into ALK receptor tyrosine kinase in human cancer biology. Nat Rev Cancer. 2013;13(10):685–700. 10.1038/nrc3580 [DOI] [PubMed] [Google Scholar]
- 23. Minoo P, Wang HY. ALK‐immunoreactive neoplasms. Int J Clin Exp Pathol. 2012;5(5):397–410. [PMC free article] [PubMed] [Google Scholar]
- 24. Fletcher CD. The evolving classification of soft tissue tumours: an update based on the new WHO classification. Histopathology. 2006;48:3–12. 10.1111/j.1365-2559.2005.02284.x [DOI] [PubMed] [Google Scholar]
- 25. Gleason BC, Hornick JL. Inflammatory myofibroblastic tumours: where are we now? J Clin Pathol. 2008;61:428–37. [DOI] [PubMed] [Google Scholar]
- 26. Coffin CM, Hornick JL, Fletcher CD. Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol. 2007;31:509–20. [DOI] [PubMed] [Google Scholar]
- 27. Sagar AES, Jimenez CA, Shannon VR. Clinical and histopathologic correlates and management strategies for inflammatory myofibroblastic tumor of the lung. A case series and review of the literature. Med Oncol. 2018;35:102. 10.1007/s12032-018-1161-0 [DOI] [PubMed] [Google Scholar]
- 28. Kovach SJ, Fischer AC, Katzman PJ, Salloum RM, Ettinghausen SE, Madeb R, et al. Inflammatory myofibroblastic tumors. J Surg Oncol. 2006;94:385–91. [DOI] [PubMed] [Google Scholar]
- 29. McDermott M. Inflammatory myofibroblastic tumour. Semin Diagn Pathol. 2016;33(6):358–66. [DOI] [PubMed] [Google Scholar]
- 30. Venizelos I, Papathomas T, Anagnostou E, Tsanakas J, Kirvassilis F, Kontzoglou G. Pediatric inflammatory myofibroblastic tumor of the trachea: a case report and review of the literature. Pediatr Pulmonol. 2008;43(8):831–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on request from the authors.


