Abstract
OBJECTIVE
Type 1 diabetes mellitus (T1DM) is a rare, irreversible immune-related adverse event reported in patients receiving treatment with immune checkpoint inhibitors (ICI). However, clinical risk factors for ICI-induced T1DM (ICI-T1DM) and its impact on survival in patients remain unknown.
RESEARCH DESIGN AND METHODS
We used Optum’s Clinformatics Data Mart database for assessment of the incidence and characteristics of T1DM in a large de-identified cohort of patients treated with ICI between 2017 and 2020. We applied Fine-Gray and cause-specific hazard models to study associations between patient/treatment characteristics and ICI-T1DM and applied the Cox model with ICI-T1DM as a time-varying covariate to assess the impact of ICI-T1DM on survival.
RESULTS
ICI-T1DM was observed in 261 of 30,337 (0.86%) patients. Dual use of antibodies to cytotoxic T lymphocyte antigen 4 (CTLA-4) and programmed cell death 1 (PD-1) or programmed cell death ligand 1 (PD-L1) was associated with increasing risk of ICI-T1DM (hazard ratio [HR] 1.62; 95% CI 1.15–2.26) vs. anti–PD-L1 or anti–PD-1 alone. Younger age (HR 1.19 for every 5-year decrease; 95% CI 1.13–1.25) and preexisting non-T1DM diabetes (HR 4.48; 95% CI 3.45–5.83) were also associated with higher risk of ICI-T1DM. Conversely, prior use of immunosuppressive medications (HR 0.57; 95% CI 0.34–0.95) was associated with lower incidence of ICI-T1DM, but part of its protective effect may be due to the increased mortality rate. Development of ICI-T1DM does not seem to significantly impact patient survival.
CONCLUSIONS
The risk of ICI-T1DM is associated with the type of ICI therapy, patient age, and preexisting non-T1DM diabetes. These data may help guide risk assessment and screening practices for patients during ICI therapy.
Introduction
Immune checkpoint inhibitors (ICI), including anti–cytotoxic T lymphocyte antigen 4 (CTLA-4), anti–programmed cell death 1 (PD-1), and anti–programmed cell death ligand 1 (PD-L1) antibodies, have revolutionized cancer treatment. Treatment with ICI therapy has improved survival in multiple malignancies, including previously treatment refractory cancers such as melanoma and lung cancer. ICI release the brakes on the immune system, allowing immune cells to detect and destroy tumor cells. With reactivation of T cells, however, ICI are associated with many immune-related adverse events, including autoimmune-like disorders. Autoimmune endocrinopathies associated with ICI have been reported in up to 4–30% of patients, with thyroid disorders being the most common (1–4). ICI-induced type 1 diabetes mellitus (ICI-T1DM) is a rare, but potentially life-threatening complication that occurs in 0.6–1.4% of patients receiving ICI (5–7).
ICI-T1DM is characterized by rapid β-cell destruction, which can occur as early as 5 days after ICI initiation and up to several months after ICI discontinuation (8–11). Of patients diagnosed with ICI-T1DM, 40–76% present with diabetic ketoacidosis (DKA), often requiring intensive care unit treatment (5,8,9,11–14), and almost all will require lifelong insulin therapy (8,9,12). To date, the focus of most studies with identification of risk factors for developing ICI-T1DM has been on clinical and laboratory data, such as HLA haplotype and autoantibody presence, which are typically not available to the clinician at the time of ICI initiation (10,12,15); in other studies investigators have relied on pooled data from ICI randomized controlled trials, which exclude patients at potentially higher risk of adverse events (16). To address these gaps, we used a national insurance claims database for assessment of incidence of ICI-T1DM, clinically available characteristics of patients with ICI-T1DM, and impact on survival of ICI-T1DM in a large cohort of patients treated with ICI.
Research Design and Methods
Database and Data Extraction
We studied a de-identified cohort of Optum’s Clinformatics Data Mart, which captures a privately insured population from a diverse group of health plans in the U.S. We included patients who were treated with pembrolizumab, nivolumab, atezolizumab, durvalumab, avelumab, cemiplimab, or ipilimumab between 2017 and 2020 and who had at least 12 months of medical records available prior to the start of ICI. We identified patients with T1DM based on ICD-10 diagnosis codes under the category of E10. We first removed patients who had at least one ICD-10 code of T1DM prior to the start of ICI and then identified patients who had ICI-T1DM based on two outpatient ICD-10 codes of T1DM at least 30 days apart or one inpatient ICD-10 code of T1DM occurring after ICI therapy (17). The onset date of T1DM was defined as the earliest date of all ICD-10 codes of T1DM. Patients who did not die were censored at the date of last follow-up. Patient characteristics recorded included age; sex; race; smoking status (former and current smoker vs. nonsmoker); ICI type (anti–CTLA-4, anti–PD-L1/anti–PD-1, or anti–CTLA-4 + anti–PD-1/anti–PD-L1); prior use of immunosuppressant medications, including traditional and biologic disease-modifying antirheumatic drug and glucocorticoids (Supplementary Table 1) within 3 months prior to the start of ICI; and Elixhauser Comorbidity Index (CMI) score (18), calculated with use of diagnosis codes in the 12-month history before ICI treatment. Specifically, we defined a combination of anti–CTLA-4 and anti–PD1/PD-L1 if both treatments were administered before ICI-T1DM for patients with ICI-T1DM, or before the last follow up for patients without ICI-T1DM, regardless of the proximity or order in which they were prescribed. We defined immunosuppressive drugs as traditional and biologic disease-modifying antirheumatic drug or glucocorticoids at supraphysiological doses (see Supplementary Table 2) for at least 30 days (19). Historically, the immunosuppressive effects of steroids are dose dependent, but it is not entirely clear whether glucocorticoids at lower doses have some immunosuppressive effects. Therefore, for differentiation of this group from the group not taking immunosuppressants, patients taking glucocorticoids at lower doses, or for shorter periods of time, were grouped as a separate category (“low-dose glucocorticoids”).
Statistical Analysis
Clinical characteristics were reported by ICI-TIDM status with categorical variables expressed as frequency and continuous variables expressed as median (range). Fine-Gray (FG) (20) and cause-specific hazard (CS) models were used to assess effects of patient/treatment characteristics on the risk of ICI-T1DM. The FG model estimates the effect of covariates on the subdistribution hazard function. Hence, significant effects of the covariates in this model can be interpreted as influencing the cumulative incidence function. The CS model estimates the effect of covariates on the hazard rate of ICI-T1DM in subjects who are still alive. The FG and CS models are based on different model assumptions and provide different interpretations. The two analyses complement each other. We implemented the CS model using the conventional Cox proportional hazards model by treating death as censoring and the FG model using cmprsk package in R. Time-varying Cox model (ICI-T1DM as a time-varying covariate and other patient characteristics as time-fixed covariates) was used to evaluate the impact of ICI-T1DM on patient survival (21). Missing data were assumed to be random and were excluded or considered as a separate category in the analyses. Statistical significance is determined with P value <0.05.
Results
Incidence and Presentation of ICI-T1DM
Based on the entry criteria, we identified 30,337 patients treated with ICI between 2017 and 2020, with a median follow-up time of 308 days. ICI-T1DM was observed in 261 patients (0.86%). The median time from ICI initiation to T1DM diagnosis was 10 weeks (range 1–95). Of the 261 patients, 78 (29.9%) presented with DKA, 76 (97.4%) of whom were hospitalized. Among the 97 patients with no prior history of diabetes, and who were thus unlikely to be checking blood glucose levels on a regular basis prior to ICI-T1DM diagnosis, 47 (48.4%) presented with DKA. Additionally, eight (3.1%) patients presented with pancreatitis, all of whom were hospitalized.
Factors Associated With ICI-T1DM
On univariate analysis based on the FG model (Table 1), Black race (hazard ratio [HR] 1.53; 95% CI 1.07–2.19) and preexisting diagnosis of other types of diabetes, including type 2 diabetes mellitus (T2DM) and ketosis-prone diabetes (HR 5.28; 95% CI 3.91–7.12), were associated with a higher incidence of ICI-T1DM. Combination therapy (anti–CTLA-4 + anti–PD-1/PD-L1) increased the risk of ICI-T1DM compared with anti–PD-1/PD-L1 monotherapy (HR 1.93; 95% CI 1.40–2.66). Younger age (HR 1.12 for every 5-year decrease; 95% CI 1.08–1.18) was also associated with higher risk of ICI-T1DM. Conversely, a higher comorbidity index (≥5 vs <5: HR 0.48; 95% CI 0.28–0.83) was associated with lower risk of ICI-TIDM, and prior use of immunosuppressive drugs (HR 0.61; 95% CI 0.27–1.00) was marginally associated with lower risk of ICI-TIDM. Conclusions in the CS model were the same (data not shown).
Table 1.
Univariate FG model for effects of patient/treatment characteristics on incidence of ICI-T1DM
| T1DM | FG model | |||
|---|---|---|---|---|
| Yes (n = 261) | No (n = 30,076) | HR (95% CI) | P | |
| Age, years, median (range) | 70 (19–89) | 72 (4–90) | 0.89 (0.85, 0.93)* | <0.0001 |
| ≤60 | 52 | 4,317 | Reference | |
| >60 | 209 | 25,759 | 0.65 (0.48, 0.88) | 0.006 |
| Tumor type | ||||
| Melanoma | 39 | 4,042 | Reference | |
| Lung | 124 | 15,711 | 0.81 (0.56, 1.16) | 0.25 |
| Both | 14 | 1,398 | 1.04 (0.57, 1.92) | 0.89 |
| Other | 84 | 8,808 | 1.00 (0.69, 1.47) | 0.99 |
| NA | 117 | 0 | ||
| ICI types | ||||
| Anti–PD-1/PD-L1 | 215 | 27,133 | Reference | |
| Anti–CTLA-4 | 1 | 83 | 1.50 (0.21, 10.69) | 0.69 |
| Anti–PD-1/PD-L1 + anti–CTLA-4 | 45 | 2,880 | 1.93 (1.40, 2.66) | 0.0001 |
| Sex | ||||
| Male | 151 | 16,846 | Reference | |
| Female | 110 | 13,225 | 0.93 (0.73, 1.19) | 0.55 |
| NA | 0 | 5 | ||
| Race | ||||
| White | 162 | 19,970 | Reference | |
| Black | 37 | 2,968 | 1.53 (1.07, 2.19) | 0.019 |
| Asian | 8 | 708 | 1.41 (0.69, 2.86) | 0.35 |
| Hispanic | 20 | 2,005 | 1.25 (0.78, 1.98) | 0.35 |
| NA | 34 | 4,425 | ||
| Weighted CMI | ||||
| <5 | 14 | 795 | Reference | |
| ≥5 | 233 | 28,552 | 0.48 (0.28, 0.83) | 0.008 |
| NA | 34 | 4,425 | ||
| Diabetes before ICI | ||||
| No | 97 | 21,339 | Reference | |
| Yes | 150 | 8,008 | 5.28 (3.91, 7,12) | <0.0001 |
| NA | 14 | 729 | ||
| Smoking | ||||
| No | 64 | 8,483 | Reference | |
| Yes | 197 | 21,593 | 1.19 (0.90, 1.58) | 0.23 |
| Immunosuppressant† | ||||
| No | 214 | 22,517 | Reference | |
| Low-dose glucocorticoids | 30 | 4,584 | 0.70 (0.48, 1.03) | 0.072 |
| Immunosuppressive drugs | 17 | 2,975 | 0.61 (0.37, 1.00) | 0.051 |
Denotes the HR for every 5-year increase in age.
Use of immunosuppressant medications within 3 months prior to the start of ICI. NA represents missing data.
In multivariable analysis with the FG model, younger age (HR 1.19 for every 5-year increase; 95% CI 1.13–1.25), prior diagnosis of other forms of diabetes (HR 4.48; 95% CI 3.45–5.83), and use of combination ICI therapy (comparing anti–CTLA-4 + anti–PD-1/PD-L1 with anti–PD-1/PD-L1) (HR 1.71; 95% CI 1.23–2.39) remained associated with higher risk of ICI-T1DM (Table 2). Prior use of immunosuppressive drugs was associated with lower risk (HR 0.57; 95% CI 0.34–0.95) of ICI-T1DM, while use of low-dose glucocorticoids seems to be associated with a reduced risk of ICI-T1DM, but it did not reach statistical significance (HR 0.72; 95% CI 0.49–1.06).
Table 2.
Multivariable FG and CS models for effects of patient/treatment characteristics on incidence of ICI-T1DM
| FG model | CS model | |||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age (unit = 5 years) | 0.84 (0.80, 0.89) | <0.0001 | 0.85 (0.80, 0.90)* | <0.0001 |
| ICI types | ||||
| Anti–PD-1/PD-L1 | Reference | Reference | ||
| Anti–CTLA-4 | 1.46 (0.21, 10.33) | 0.70 | 1.40 (0.20, 10.07) | 0.74 |
| Anti–PD-1/PD-L1 + anti–CTLA-4 | 1.71 (1.23, 2.39) | 0.0016 | 1.61 (1.15, 2.26) | 0.0055 |
| Sex | ||||
| Male | Reference | Reference | ||
| Female | 1.05 (0.81, 1.35) | 0.74 | 1.02 (0.79, 1.32) | 0.88 |
| Race/ethnicity | ||||
| White | Reference | Reference | ||
| Black | 1.25 (0.87, 1.81) | 0.23 | 1.25 (0.86, 1.81) | 0.24 |
| Asian | 1.10 (0.51, 2.35) | 0.81 | 1.09 (0.51, 2.33) | 0.83 |
| Hispanic | 1.03 (0.64, 1.67) | 0.89 | 1.04 (0.64, 1.68) | 0.87 |
| Weighted CMI | ||||
| <5 | Reference | Reference | ||
| ≥5 | 0.61 (0.35, 1.05) | 0.072 | 0.69 (0.40, 1.18) | 0.17 |
| Diabetes before ICI | ||||
| No | Reference | Reference | ||
| Yes | 4.48 (3.45, 5.83) | <0.0001 | 4.66 (3.58, 6.06) | <0.0001 |
| Smoking | ||||
| No | Reference | Reference | ||
| Yes | 1.27 (0.94, 1.72) | 0.12 | 1.29 (0.96, 1.74) | 0.091 |
| Immunosuppressant† | ||||
| No | Reference | Reference | ||
| Low-dose glucocorticoids | 0.72 (0.49, 1.06) | 0.091 | 0.75 (0.51, 1.10) | 0.15 |
| Immunosuppressive drugs | 0.57 (0.34, 0.95) | 0.031 | 0.67 (0.40, 1.11) | 0.12 |
Use of immunosuppressant medication within 3 months prior to the start of ICI.
Age, prior diagnosis of other forms of diabetes, and use of combination ICI therapy remained significant in the CS model, indicating that these factors influenced both the incidence and CS of ICI-T1DM. However, the effect of prior use of immunosuppressive drugs on risk of ICI-T1DM became nonsignificant (HR 0.67; 95% CI 0.40–1.11). As we found that prior use of immunosuppressive drugs was associated with a higher mortality rate (HR 1.67; 95% CI 1.59–1.76 [based on CS model]), different results from the FG and CS models suggest that part of the protective effect of the prior use of immunosuppressive drugs on diagnosis of ICI-T1DM is due to the increased mortality rate, preventing ICI-T1DM from being observed.
ICI-T1DM and Survival
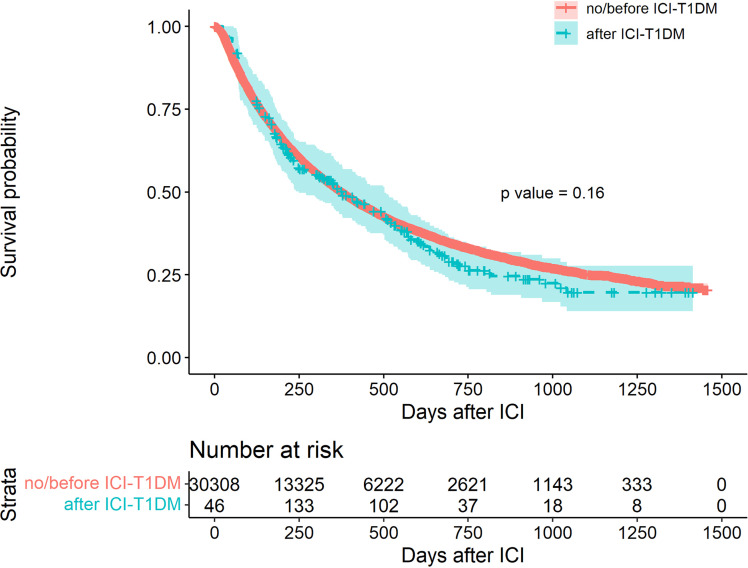
Of the 30,337 patients, 15,359 (50.63%) died during 2017–2020, and of these, 143 died after diagnosis of ICI-T1DM. Occurrence of T1DM following ICI had no significant impact on overall survival in univariate (Fig. 1) or multivariable analysis after adjustment for age, sex, race, cancer type, ICI type, CMI, smoking status, and prior use of immunosuppressants (Table 3).
Figure 1.
Kaplan-Meier curves for overall survival (ICI-TIDM is a time-varying covariate).
Table 3.
Multivariable time-varying Cox regression for effect of ICI-T1DM on overall survival
| HR (95% CI) | P | |
|---|---|---|
| T1DM | 1.14 (0.95, 1.37) | 0.16 |
| Age (unit = 5 years) | 1.07 (1.06, 1.08) | <0.0001 |
| ICI types | ||
| Anti–PD-1/PD-L1 | Reference | |
| Anti–CTLA-4 | 0.73 (0.49, 1.08) | 0.11 |
| Anti–PD-1/PD-L1 + anti–CTLA-4 | 0.89 (0.84, 0.95) | 0.0003 |
| Tumor type | ||
| Melanoma | Reference | |
| Lung | 1.70 (1.59, 1.81) | <0.0001 |
| Both | 1.70 (1.55, 1.87) | <0.0001 |
| Other | 1.81 (1.70, 1.93) | <0.0001 |
| Sex | ||
| Male | Reference | |
| Female | 0.89 (0.86, 0.92) | <0.0001 |
| Race | ||
| White | Reference | |
| Black | 0.99 (0.94, 1.07) | 0.64 |
| Asian | 0.98 (0.88, 1.09) | 0.69 |
| Hispanic | 0.94 (0.88, 1.01) | 0.07 |
| Weighted CMI | ||
| <5 | Reference | |
| ≥5 | 1.53 (1.36, 1.73) | <0.0001 |
| Diabetes before ICI | ||
| No | Reference | |
| Yes | 1.12 (1.08, 1.16) | <0.0001 |
| Smoking | ||
| No | Reference | |
| Yes | 1.13 (1.08, 1.18) | <0.0001 |
| Immunosuppressant† | ||
| No | Reference | |
| Low-dose glucocorticoids | 1.21 (1.15, 1.27) | <0.0001 |
| Immunosuppressive drugs | 1.63 (1.55, 1.72) | <0.0001 |
Use of immunosuppressant medication within 3 months prior to the start of ICI.
Older age (HR 1.07; 95% CI 1.06–1.08), male sex (HR 1.12; 95% CI 1.08–1.16), higher CMI (>5 vs. ≤5, HR 1.53; 95% CI 1.36–1.73), smoking (HR 1.13; 95% CI 1.08–1.18), lung cancer (HR 1.70; 95% CI 1.59–1.81), prior use of immunosuppressive drugs (HR 1.63; 95% CI 1.55–1.72), use of low-dose glucocorticoids (HR 1.21; 95% CI 1.15–1.27), and presence of other types of diabetes prior to ICI therapy (HR 1.12; 95% CI 1.08–1.16) were associated with shorter survival.
Conclusions
To our knowledge, this is the largest study of ICI-induced T1DM and the first using U.S.-based insurance claims data to evaluate potential risk factors that would be available at the time of ICI treatment initiation. Insurance claims data present a cost-effective way to study rare adverse events such as ICI-T1DM, providing tremendous analytical flexibility that is not possible even with large epidemiologic cohorts. We found that the risk of ICI-T1DM is low (0.86%), and >30% patients with ICI-T1DM present with serious acute complications, such as DKA or pancreatitis, and both complications often require hospitalization.
Our study found that dual ICI therapy increased the risk of developing T1DM-ICI, which is consistent with prior case reports of patients who developed fulminant diabetes after receiving both anti–CTLA-4 and anti–PD-1/anti–PD-L1 treatment (8,9,22). While anti–PD-1 therapy has previously been linked to ICI-T1DM, less is known about dual ICI use. PD-1 promotes islet-specific tolerance, while PD-1 inactivation has been linked to autoreactive T-cell activation in mouse models of autoimmune diabetes (23,24). CTLA-4 has not been linked with β-cell tolerance or T1DM specifically but, rather, affects T-cell activation through a pathway different from that of PD-1 (25,26). As anti–PD-1 and anti–CTLA-4 therapies target different components of the T-cell response, dual use of both types of therapy may lead to a two-hit model in which CTLA-4 inhibition increases T-cell activation and PD-1 inhibition leads to β-cell–specific targeting.
The average age of patients who developed ICI-T1DM in our analysis was 68 years, consistent with prior studies. As others have noted, age at diagnosis of ICI-T1DM is significantly higher than what is typically seen in T1DM, likely reflecting the patient population that receives ICI (8,10–12). Because our data set includes a large population with a wide range of ages (4–90 years), we were able to identify younger age as a risk factor for developing ICI-T1DM, which has not previously been shown. While it is unclear why younger age increases risk for ICI-T1DM, this finding may be an important part of risk assessment in initiating ICI.
The results of our analysis demonstrate that patients with a history of T2DM and other types of diabetes prior to ICI initiation had an increased risk of developing ICI-T1DM, as previously suggested in several case reports (9). While the underlying cause of T2DM is insulin resistance, patients with long-standing T2DM have decreased β-cell mass and β-cell failure (27–29). Similarly, most diagnoses coded within E13 (“other specified diabetes mellitus”) are due to non-immune-mediated insulin deficiency. Thus, even a small amount of ICI-mediated β-cell destruction may result in profound insulin deficiency, DKA, and de novo insulin requirement in patients with underlying T2DM or other forms of diabetes. Others have hypothesized that some patients with a history of T2DM who subsequently develop ICI-T1DM may have latent autoimmune diabetes of adults with anti-GAD antibodies, predisposing them ICI-T1DM (30). While worsening of T2DM and other types of diabetes could conceivably be miscoded as T1DM, our entry criterion of two T1DM codes 30 days apart makes this less likely.
In contrast to early reports identifying White race to be associated with the highest risk of ICI-T1DM (11,31), we found that ICI-T1DM was more likely to occur in the case of Black race in our univariate analysis. However, after controlling for confounders, such as age, sex, and prior history of T2DM, this association was no longer significant. T2DM is more common in African Americans in the U.S. (32), and we also observed an increased risk of ICI-T1DM in patients with a history of T2DM. Therefore, the high prevalence of T2DM for Black race is the likely explanation for the significant finding in the unadjusted univariate analysis.
Another finding in our study is that prior immunosuppressive therapy decreased the risk of developing ICI-T1DM. With further analysis we found that part of the reason for this protective effect might be the increased mortality rate. To our knowledge, this is the first effort to explore the role of prior immunosuppression in this patient population. Our observation that the increased risk of prior immunosuppressive therapy on the mortality rate may be due to either the effects of immunosuppressive therapies themselves, or the underlying disease that immunosuppressive therapies are treating, such as rheumatologic, pulmonary, gastrointestinal or endocrine disorders. However, identifying a specific autoimmune disease based on ICD-10 codes is challenging; therefore, additional studies will be needed to determine the mechanism through which a history of prior immunosuppressive therapy leads to increased mortality rate.
Study limitations include dependence on ICD-10 codes and lack of information on severity of adverse events and tumor stage. Due to these limitations, studying the effect of ICI-T1DM on patient survival is challenging, as survival is related to the severity of ICI-TIDM and cancer stage. Identifying a specific autoimmune disease based on ICD-10 codes is challenging; therefore, it is hard to tell whether the significant association between prior use of immunosuppressants and poor survival is due to the effect of medication or due to the underlying autoimmune disease that is not captured in our data.
Despite these limitations, our data provide important insights into ICI-T1DM. While the incidence of ICI-T1DM is low, the occurrence of DKA and pancreatitis can be life-threatening. Early identification through monitoring symptoms and glucose levels is important. Health care providers must be aware of this new form of T1DM in patients treated with ICI, and patient education on early hyperglycemia symptoms, such as hunger, thirst, and frequent urination, should be provided in high-risk populations prior to ICI initiation. HLA genotypes and other patient characteristics possibly serving as predictive biomarkers are the subject of ongoing studies.
Article Information
Acknowledgments. The authors acknowledge the Data and Methods Hub at the University of Michigan Institute for Healthcare Policy and Innovation for helping with Optum’s Clinformatics Data Mart database.
Funding. This work was supported by the National Institutes of Health (grant P30 CA 046592).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. X.C., A.H.A., and L.Z. wrote the first draft. X.C. and L.Z. performed the statistical analyses. X.C., A.H.A., Y.L., and L.Z. collected the data and performed quality control. X.C., A.H.A., A.F.T., N.L.H., E.S., A.Q., M.O., D.C., N.R., and L.Z. provided expert interpretation of the findings. All co-authors reviewed the initial draft and provided critical revisions. All authors approved the final version of the manuscript. L.Z. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
X.C. and A.H.A. made equal contributions.
This article contains supplementary material online at https://doi.org/10.2337/figshare.19165250.
References
- 1. Barroso-Sousa R, Barry WT, Garrido-Castro AC, et al. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncol 2018;4:173–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 2018;378:158–168 [DOI] [PubMed] [Google Scholar]
- 3. Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front Oncol 2018;8:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sznol M, Postow MA, Davies MJ, et al. Endocrine-related adverse events associated with immune checkpoint blockade and expert insights on their management. Cancer Treat Rev 2017;58:70–76 [DOI] [PubMed] [Google Scholar]
- 5. Kotwal A, Haddox C, Block M, Kudva YC. Immune checkpoint inhibitors: an emerging cause of insulin-dependent diabetes. BMJ Open Diabetes Res Care 2019;7:e000591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Perdigoto AL, Quandt Z, Anderson M, Herold KC. Checkpoint inhibitor-induced insulin-dependent diabetes: an emerging syndrome. Lancet Diabetes Endocrinol 2019;7:421–423 [DOI] [PubMed] [Google Scholar]
- 7. Shimada K, Yamamoto H, Nakatani E, et al. Real-world evidence of the incidence of and risk factors for type 1 diabetes mellitus and hypothyroidism as immune-related adverse events associated with programmed cell death-1 inhibitors. Endocr Pract 2021;27:586–593 [DOI] [PubMed] [Google Scholar]
- 8. Akturk HK, Kahramangil D, Sarwal A, Hoffecker L, Murad MH, Michels AW. Immune checkpoint inhibitor-induced type 1 diabetes: a systematic review and meta-analysis. Diabet Med 2019;36:1075–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stamatouli AM, Quandt Z, Perdigoto AL, et al. Collateral damage: insulin-dependent diabetes induced with checkpoint inhibitors. Diabetes 2018;67:1471–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tsang VHM, McGrath RT, Clifton-Bligh RJ, et al. Checkpoint inhibitor-associated autoimmune diabetes is distinct from type 1 diabetes. J Clin Endocrinol Metab 2019;104:5499–5506 [DOI] [PubMed] [Google Scholar]
- 11. Wright JJ, Salem J-E, Johnson DB, et al. Increased reporting of immune checkpoint inhibitor-associated diabetes. Diabetes Care 2018;41:e150–e151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dougan M, Pietropaolo M. Time to dissect the autoimmune etiology of cancer antibody immunotherapy. J Clin Invest 2020;130:51–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Imagawa A, Hanafusa T, Miyagawa J; Osaka IDDM Study Group . A novel subtype of type 1 diabetes mellitus characterized by a rapid onset and an absence of diabetes-related antibodies. N Engl J Med 2000;342:301–307 [DOI] [PubMed] [Google Scholar]
- 14. Shiba M, Inaba H, Ariyasu H, et al. Fulminant type 1 diabetes mellitus accompanied by positive conversion of anti-insulin antibody after the administration of anti-CTLA-4 antibody following the discontinuation of anti-PD-1 antibody. Intern Med 2018;57:2029–2034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marchand L, Thivolet A, Dalle S, et al. Diabetes mellitus induced by PD-1 and PD-L1 inhibitors: description of pancreatic endocrine and exocrine phenotype. Acta Diabetol 2019;56:441–448 [DOI] [PubMed] [Google Scholar]
- 16. Lu J, Yang J, Liang Y, Meng H, Zhao J, Zhang X. Incidence of immune checkpoint inhibitor-associated diabetes: a meta-analysis of randomized controlled studies. Front Pharmacol 2019;10:1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kehl KL, Yang S, Awad MM, Palmer N, Kohane IS, Schrag D. Pre-existing autoimmune disease and the risk of immune-related adverse events among patients receiving checkpoint inhibitors for cancer. Cancer Immunol Immunother 2019;68:917–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moore BJ, White S, Washington R, Coenen N, Elixhauser A. Identifying increased risk of readmission and in-hospital mortality using hospital administrative data: the AHRQ Elixhauser Comorbidity Index. Med Care 2017;55:698–705 [DOI] [PubMed] [Google Scholar]
- 19. Nicolaides NC, Pavlaki AN, Alexandra MAM, et al. Glucocorticoid therapy and adrenal suppression. In Endoxtext. Feingold KR, Anawalt B, Boyce A, et al., Eds. South Dartmouth, MA, MDText.com, Inc., 2015 [Google Scholar]
- 20. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509 [Google Scholar]
- 21. Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response and other comparisons of time-to-event by outcome variables. J Clin Oncol 2008;26:3913–3915 [DOI] [PubMed] [Google Scholar]
- 22. Zezza M, Kosinski C, Mekoguem C, et al. Combined immune checkpoint inhibitor therapy with nivolumab and ipilimumab causing acute-onset type 1 diabetes mellitus following a single administration: two case reports. BMC Endocr Disord 2019;19:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ansari MJ, Salama AD, Chitnis T, et al. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med 2003;198:63–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Martinov T, Spanier JA, Pauken KE, Fife BT. PD-1 pathway-mediated regulation of islet-specific CD4+ T cell subsets in autoimmune diabetes. Immunoendocrinology (Houst) 2016;3:e1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Buchbinder EI, Desai A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol 2016;39:98–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rui J, Deng S, Arazi A, Perdigoto AL, Liu Z, Herold KC. β cells that resist immunological attack develop during progression of autoimmune diabetes in NOD mice. Cell Metab 2017;25:727–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. β-Cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes 2003;52:102–110 [DOI] [PubMed] [Google Scholar]
- 28. Clark A, Wells CA, Buley ID, et al. Islet amyloid, increased A-cells, reduced B-cells and exocrine fibrosis: quantitative changes in the pancreas in type 2 diabetes. Diabetes Res 1988;9:151–159 [PubMed] [Google Scholar]
- 29. Rahier J, Guiot Y, Goebbels RM, Sempoux C, Henquin J-C. Pancreatic β-cell mass in European subjects with type 2 diabetes. Diabetes Obes Metab 2008;10(Suppl. 4):32–42 [DOI] [PubMed] [Google Scholar]
- 30. Akturk HK, Alkanani A, Zhao Z, Yu L, Michels AW. PD-1 inhibitor immune-related adverse events in patients with preexisting endocrine autoimmunity. J Clin Endocrinol Metab 2018;103:3589–3592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Quandt Z, Young A, Anderson M. Immune checkpoint inhibitor diabetes mellitus: a novel form of autoimmune diabetes. Clin Exp Immunol 2020;200:131–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Centers for Disease Control and Prevention . National Diabetes Statistics Report. Accessed 12 October 2021. Available from https://www.cdc.gov/diabetes/library/features/diabetes-stat-report.html
- 33. Bresson D, von Herrath M. Immunotherapy for the prevention and treatment of type 1 diabetes: optimizing the path from bench to bedside. Diabetes Care 2009;32:1753–1768 [DOI] [PMC free article] [PubMed] [Google Scholar]