Scheme 3.
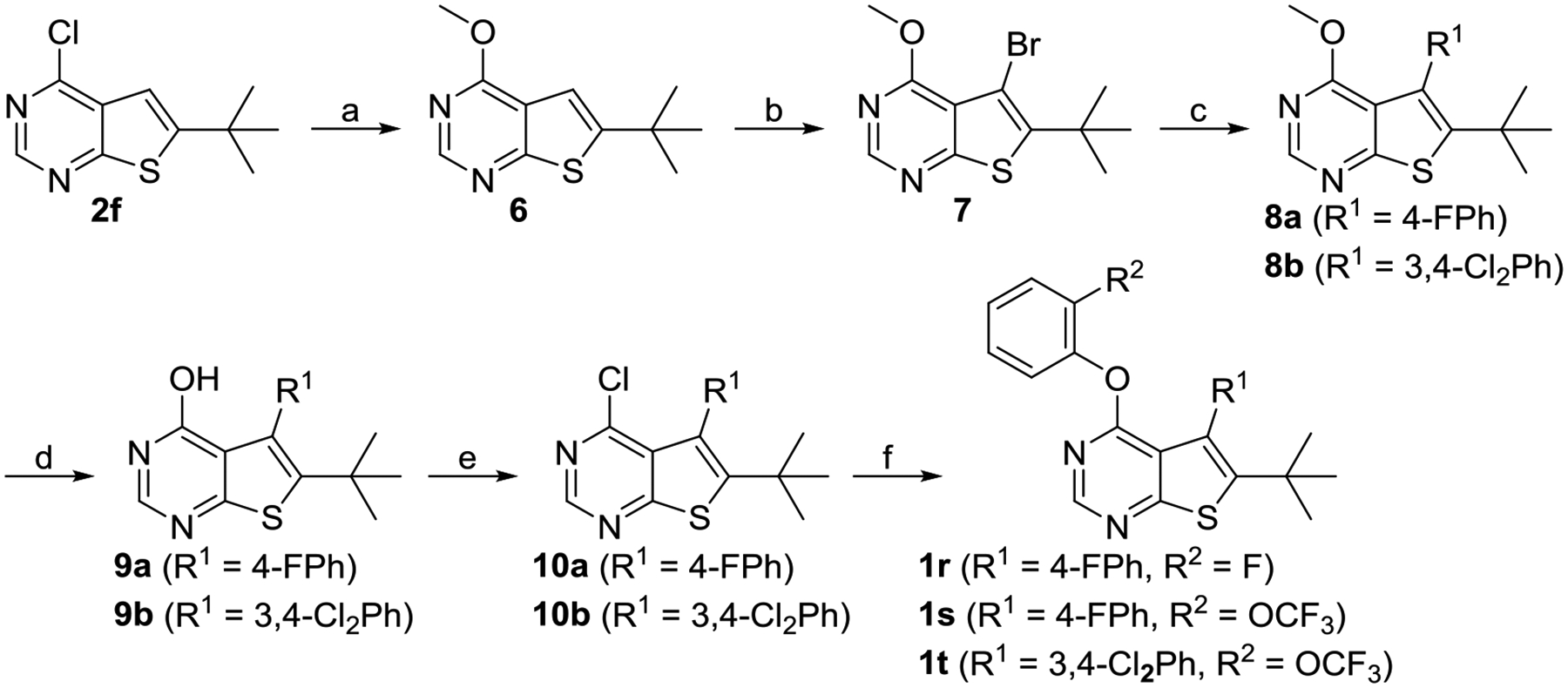
Synthesis of Compounds 1r-ta
aReagents and conditions: (a) MeONa, MeOH, 0 °C to rt, 98%; (b) NBS, AcOH, 55 °C, 85%; (c) R1B(OH)2, PdCl2(PPh3)2, K2CO3, DMF, 80 °C, 92% for 8a, 75% for 8b; (d) BBr3, DCM, rt, 41% for 9a, 93% for 9b; (e) POCl3, 110 °C, 61% for 10a, 95% for 10b; (f) 2-fluorophenol or 2-(trifluoromethoxy)phenol, NaH, DMF, 0 °C to rt, 77% for 1r, 65% for 1s, 69% for 1t.
