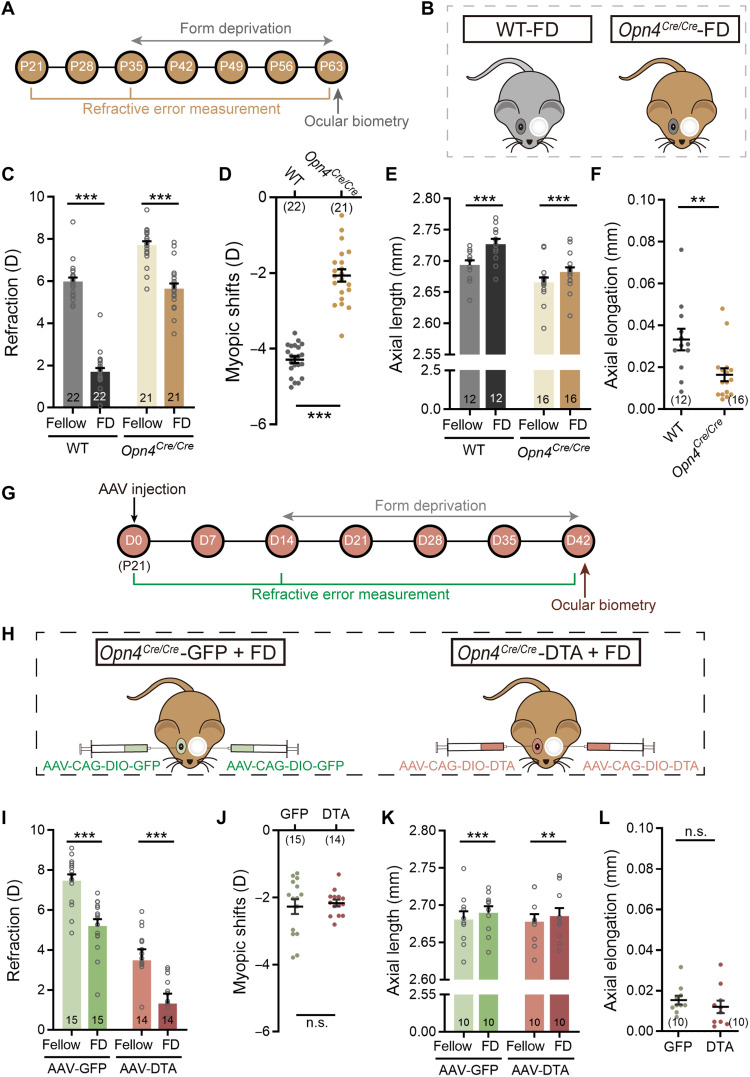
Fig. 6. Melanopsin signals mediate ipRGC contribution to FDM by promoting axial growth.
(A) Procedure/data collection timeline for the experiments shown in (B) to (F). (B) Schematic illustration of WT (left) and Opn4Cre/Cre mice (right); starting at P35, mice were form-deprived on left eyes for 4 weeks. (C) Form deprivation induced significant myopic shifts in deprived eyes as compared to fellow eyes in both WTs and Opn4Cre/Cre mice. (D) The myopic shifts induced in Opn4Cre/Cre mice were significantly smaller than those in WT mice. (E) In both genotypes, the AL of deprived eyes was significantly larger than that of fellow eyes. (F) The axial elongation in Opn4Cre/Cre mice was significantly smaller as compared to WT mice. (G) Procedure and data collection pipeline of the experiments conducted on AAV-injected Opn4Cre/Cre mice. (H) Schematic illustration of dividing Opn4Cre/Cre mice into two groups: one binocularly injected with control virus (AAV-CAG-DIO-GFP, left), and the other binocularly injected with AAV-CAG-DIO-DTA (right); both groups were treated with 4-week monocular form deprivation starting at D14 (P35). (I) In both groups, refractive errors in deprived eyes were significantly smaller than those in fellow eyes, suggesting a myopic shift. (J) Grouped data show that no significant difference in deprivation-induced myopic shifts was detected between DTA and control virus–injected Opn4Cre/Cre mice. (K) The AL of deprived eyes was significantly larger than that of fellow eyes in both groups. (L) The axial elongation in DTA virus–injected Opn4Cre/Cre mice was comparable to that in control virus–treated Opn4Cre/Cre mice after 4-week form deprivation. Error bars represent SEM. **P < 0.01, ***P < 0.001.