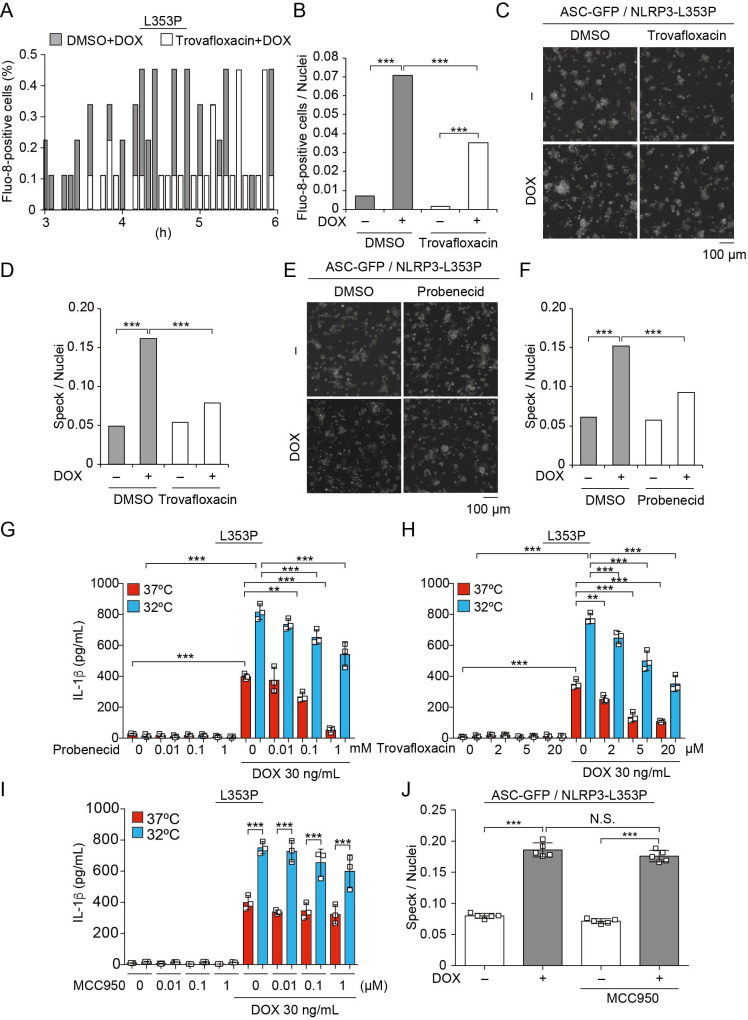
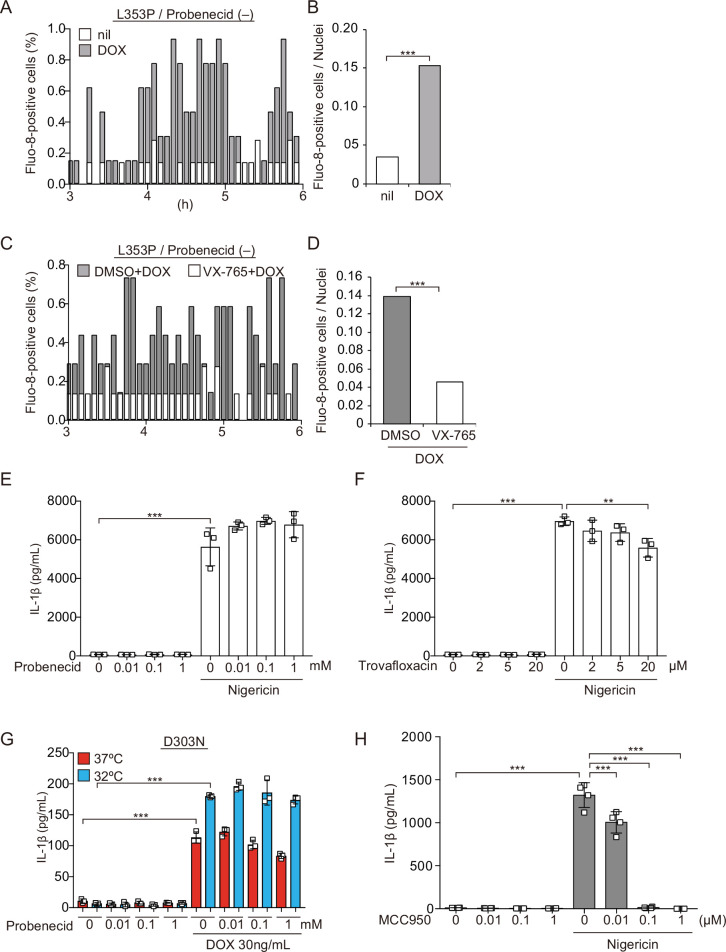
Figure 8. Pannexin 1 inhibition prevents familial cold autoinflammatory syndrome-associated NLRP3 mutant-mediated inflammasome assembly.
(A and B) Differentiated TRE-NLRP3-L353P-THP-1 cells were pretreated with 4 µM Fluo-8 for 1 hr and trovafloxacin (20 µM) for 30 min. After doxycycline (DOX) (30 ng/mL) treatment, the images were recorded at 5 min intervals from 3 hr to 6 hr. (A) The frequency of intracellular Ca2+ increase at each time point. (B) The cumulative number of Fluo-8-positive cells. (C–F) EF1-ASC-GFP/TRE-NLRP3-L353P-THP-1 cells were pretreated with (C and D) trovafloxacin (20 µM) or (E and F) probenecid (1 mM) for 30 min and then treated with DOX (30 ng/mL) at 37°C for 6 hr. Apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC)-speck formation was analyzed by confocal microscopy. (C and E) Representative images by confocal microscopy. (D and F) The number of nuclei and specks was counted. (G – I) Differentiated TRE-NLRP3-L353P-THP-1 cells were pretreated with (G) trovafloxacin, (H) probenecid, or (I) MCC950 for 30 min and then treated with DOX (30 ng/mL) at 37 or 32°C for 6 hr. The IL-1β levels in the supernatants were assessed by ELISA (n=3). (J) EF1-ASC-GFP/TRE-NLRP3-L353P-THP-1 cells were pretreated with MCC950 and then treated with DOX (30 ng/mL) at 37°C. The formation of ASC speck was analyzed by high-content analysis. (G–J) Data are expressed as the mean ± SD. **p<0.01 and ***p<0.005 as determined by (G–J) two-way ANOVA with a post hoc test or (B, D, and F) Fisher’s exact test with the Holm correction. Data are representative of three independent experiments.