Abstract
We herein report a 72-year-old woman with rheumatoid vasculitis who exhibited a depressed level of consciousness after receiving the first dose of the Pfizer-BioNTech mRNA BNT162b COVID-19 vaccine and was diagnosed with meningoencephalitis. Although there was no confirmatory examination, the diagnosis was based on magnetic resonance imaging (MRI) findings and etiological assessments, including microbiological and autoimmune investigations. Both intravenous steroid pulse and gammaglobulin therapies alleviated the patient's symptoms, and the MRI findings improved. Although the efficacy of COVID-19 vaccination has been widely accepted, such neurologic complications might occur in patients with rheumatoid diseases or vasculitis syndromes.
Keywords: Pfizer-BioNTech mRNA BNT162b COVID-19 postvaccination, meningoencephalitis, magnetic resonance imaging
Introduction
Vaccines to prevent coronavirus disease 2019 (COVID-19) infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are considered the most promising approach for controlling the pandemic. At the time of writing, two vaccines have been approved in Japan: the mRNA BNT162b2 Pfizer-BioNTech (Pfizer COVID-19 vaccine) (1) and the mRNA 1273 Moderna (2). While the safety of many of these vaccines has been assessed in clinical trials and post-marketing product surveillance, monitoring of authorized vaccines continues to highlight problems or side effects that were not originally detected.
Medical issues due to vaccines, if present, tend to emerge early in the vaccination program when they can be identified and addressed. Common systemic signs and symptoms, such as a fever, fatigue, headache, chills, myalgia and arthralgia, can occur following COVID-19 vaccination; however, most systemic postvaccination signs and symptoms are mild to moderate in severity, occur within the first three days of vaccination and resolve within one to two days of the onset (1,3). Recently, relatively serious side-effects of Pfizer COVID-19 postvaccination, such as pericarditis and myocarditis, have been reported (4,5). In addition, to our knowledge, meningoencephalitis after receiving the Pfizer COVID-19 vaccine has been reported in four cases to date (6-9). However, MRI findings were not described for these cases, and all patients were diagnosed with aseptic meningitis.
We herein describe the first reported case of acute meningoencephalitis with rheumatoid vasculitis following receipt of the first dose of the Pfizer COVID-19 vaccine.
Case Report
A 72-year-old Japanese woman presented to the emergency department with a depressed level of consciousness [Glasgow Coma Scale (GCS) E3M2V4]. She had received her first dose of the Pfizer COVID-19 vaccine three days prior to presentation. After the vaccination, she had complained of general fatigue and headache but no signs or symptoms of myalgia, arthralgia or any skin change. She had a history of rheumatoid vasculitis that had been pathologically diagnosed from a skin biopsy for the purpura as petechiae of her legs in 2015. Her serum laboratorial abnormalities at the time had included increased matrix metalloproteinase 3 (MMP-3) (304.8 ng/mL, normal 17.3-59.7), anti-cyclic citrullinated peptides (CCP) antibody (<0.6 U/mL, normal <0.6) and immunoglobulin (Ig) G-rheumatoid factor (RF) (2.4, normal <2), but she had no rheumatoid arthritis symptoms. She had continuously taken prednisolone at 6 mg daily but had no history of any other immunosuppressive medications. In addition, she had a history of diabetes mellitus and hyperlipidemia and was taking sitagliptin 50 mg daily and atorvastatin 10 mg daily.
A physical examination revealed that her vital signs were normal with blood pressure of 112/73 mmHg, heart rate of 96 beats/min and respiratory rate of 19 breaths/min. A neurological examination found no motor laterality or pathological reflexes, except for her decreased level of consciousness. Laboratory screening revealed normal values in leukocytes (7,500 cells/μL) and a slight increase in the C-reactive protein (CRP) level (3.96 mg/dL). Endocrine examinations revealed no abnormalities in the thyroid (thyroid-stimulating hormone: 1.521 μIU/mL, free-T3: 1.80 pg/mL, free-T4: 0.96 ng/dL) or adrenal gland (cortisol: 4.28 μg/dL, adrenaline: 12 pg/mL) function. Blood coagulation tests showed a slightly increased D-dimer level (2.7 μg/mL, normal <1). A nasopharyngeal swab was taken, and SARS-CoV-2 ribonucleic acid (RNA) was not detected by reverse transcription polymerase chain reaction (RT-PCR) (10). Serum levels relevant to rheumatoid vasculitis included MMP-3 of 67.4 ng/mL, anti-CCP antibody of <0.6 U/mL and IgG-RF of 1.2. Diagnostic lumbar puncture was performed, and turbid yellow cerebrospinal (CSF) fluid was collected with an opening pressure of 15 mmHg (normal 8-15 mmHg). An examination of the CSF revealed a cell count of 4 cells/mm3 (all mononuclear leukocytes), an increased protein level of 173.2 mg/dL and a normal glucose level of 72.3 mg/dL, with a plasma glucose level of 108 mg/dL. The patient's IgG index was elevated to 1.13 (normal <0.66).
At that time, this case was diagnosed as uncommon meningoencephalitis, and the patient was immediately started on both intravenous immunoglobulin (70 mg by continuous infusion for 24 hours) and methylprednisolone (1,000 mg/daily for 3 days and continuously 500 mg/daily for 2 days). The next day, her level of consciousness had improved remarkably. At a later date, culture analyses of both the blood and CSF showed no abnormalities on PCR for varicella zoster, herpes simplex or cytomegalovirus.
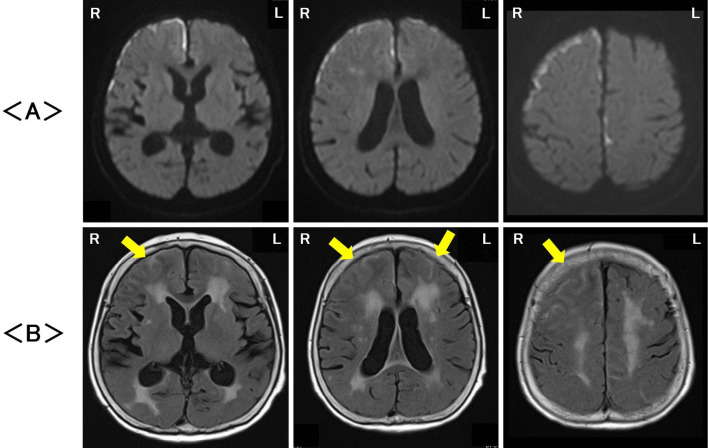
Magnetic resonance imaging (MRI; 1.5T Siemens MAGNETOM Aera, Munich, Germany) on the first hospital day revealed high signals at the surface of the cerebral cortex especially and in the white matter of the bilateral frontotemporal areas on diffusion-weighted imaging (DWI), with signals being particularly strong on the right side compared with the left side (Fig. 1A). Fluid-attenuated inversion recovery (FLAIR) images showed an abnormal signal in both the cerebral gray and white matter and diffuse cerebral cortex swelling in the bilateral frontotemporal areas, which was also stronger on the right side than on the left side (Fig. 1B). Magnetic resonance angiography did not reveal any abnormalities in the brain. Gadolinium-enhanced MRI was not performed because of her history of allergic reaction to contrast medium.
Figure 1.
Magnetic resonance imaging on the first hospital day revealed high signals, particularly on the surface of the cerebral cortex as well as in the white matter in the bilateral frontotemporal areas, with signals being stronger on the right side than on the left side on diffusion-weighted imaging (DWI) (A). Fluid-attenuated inversion recovery (FLAIR) images showed abnormal signals in both the cerebral gray and white matter, and diffuse cerebral cortex swelling (arrowhead) was noted in the bilateral frontotemporal areas, also being stronger on the right side than on the left side (B). R: right, L: left
There were no indicators of any other autoimmune disease except for rheumatoid vasculitis; serum anti-neutrophil cytoplasmic antibodies (ANCA) of both myeloperoxidase (MPO) and proteinase3 (PR3), IgG4 antibody, myelin basic protein and oligoclonal IgG bands in the CSF were observed. An electroencephalogram showed wide-range slow waves on both sides of the front-temporal areas but no form of epileptic activity. The patient's symptoms improved rapidly after the administration of intravenous immunoglobulin and methylprednisolone. No additional antibiotic, antiviral or antifungal medications were used during the in-hospital period. We did not perform a biopsy because of the high risk of perioperative complications.
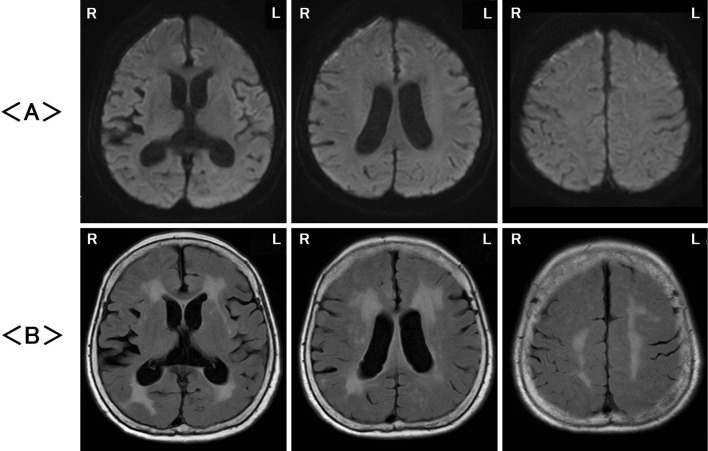
Two months later, follow-up MRI showed the disappearance of high signals at the surface of the cerebral cortex and white matter on DWI (Fig. 2A) and improvements in the abnormal signals in the cerebral gray and white matter as well as diffuse cerebral cortex swelling on FLAIR images (Fig. 2B).
Figure 2.
Magnetic resonance imaging two months later revealed no abnormalities on diffusion-weighted imaging (DWI) (A) and fluid-attenuated inversion recovery (FLAIR) images (B). R: right, L: left
Discussion
We herein report a case involving an adult patient with meningoencephalitis following the first dose of the Pfizer-BioNTech mRNA BNT162b COVID-19 vaccine that was diagnosed based on MRI findings.
Meningoencephalitis may be caused by several disorders, including viral/bacterial/fungus infections or autoimmune diseases (11). In this case, clinical and laboratory examinations showed no indications of an underlying structural cause of meningoencephalitis except for her history of rheumatoid vasculitis, suggesting that this rheumatoid disease might have been, to some extent, associated with this meningoencephalitis. The MRI findings were suggestive of rheumatoid meningoencephalitis (12,13). However, this case did not have a high activity of rheumatoid disease at that time, especially regarding anti-CCP antibodies. Rheumatoid meningoencephalitis is a rare manifestation of rheumatoid diseases, but its existence has recently become recognized. It is characterized by inflammatory CSF and radiological evidence of meningeal enhancement with or without underlying parenchymal signal changes. Many of these cases indicate that rheumatoid meningitis can co-exist with high levels of anti-CCP antibodies in both the serum and CSF (12-14).
Approximately a dozen cases of meningoencephalitis after COVID-19 infection have been reported at this time (15,16), and in some of these cases, SARS-CoV-2 has been detected in the CSF. However, our case did not result from COVID-19 infection and differed markedly from these previous reports in terms of the clinical course.
Once the Pfizer COVID-19 vaccine has been injected, the vaccine particles interact with cells and fuse to them, releasing mRNA. The cell's molecules then read the sequence and present fragments of the spike protein on their surface. When helper T cells detect these fragments, they can raise the alarm and help marshal other immune cells to fight the COVID-19 infection. Other immune agents, such as B cells, also interact with the coronavirus spikes on the surface of vaccinated cells and free-floating spike protein fragments (17). Previous reports have suggested that the molecular mimicry triggered by the protein generated through immunization can result in autoimmune meningitis, whereby the vaccine-generated S1 protein might cause a breach of the blood-brain barrier and result in aseptic meningitis (6,7).
The inflammation of rheumatoid meningoencephalitis is commonly characterized by infiltration of CD3-positive T cells, B lymphocytes, plasma cells, multinucleated giant cells, macrophages, necrotizing granulomas and fibrosis along with astrocytosis in the adjacent neural parenchyma (18,19). However, the definite pathological mechanism has not yet been elucidated. Based on our findings, we speculate that the post-COVID-19-vaccination immune system might evoke the disease state to rheumatoid meningoencephalitis.
The present case is also the first report of an adult patient with meningoencephalitis after COVID-19 vaccination that was associated with rheumatoid disease. Why neurological deficits may suddenly arise following COVID-19 vaccination remains unclear; pathological and immunological mechanisms, such as rheumatoid vasculitis via direct effects or inflammation accompanying the effects of vaccination, are commonly suggested. In the present study, we did not measure the level of anti-myelin oligodendrocyte glycoprotein (MOG) antibody, which is an indicator of acute disseminated encephalomyelitis (ADEM) (20). It is also controversial that a full recovery from meningoencephalitis can be achieved using immunoglobulins, which were not used in previous reports of the treatment of rheumatoid meningoencephalitis (12-14). The same clinical course may be observed with other types of meningoencephalitis after COVID-19 vaccination. Thus, further investigations will be required to determine the most appropriate treatment strategy for cases such as the one described in this report.
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
The authors state that they have no Conflict of Interest (COI).
Joe Senda and Ryosei Ashida contributed equally to this work.
References
- 1. Polack FP, Thomas SJ, Kitchin N, et al. ; C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med 383: 2603-2615, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baden LR, El Sahly HM, Essink B, et al. ; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 384: 403-416, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McMurry R, Lenehan P, Awasthi S, et al. Real-time analysis of a mass vaccination effort confirms the safety of FDA-authorized mRNA COVID-19 vaccines. Med (N Y) 2: 1-14, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bozkurt B, Kamat I, Hotez PJ. Myocarditis with COVID-19 mRNA vaccines. Circulation 144: 471-484, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vidula MK, Ambrose M, Glassberg H, et al. Myocarditis and other cardiovascular complications of the mRNA-based COVID-19 vaccines. Cureus 13: e15576, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ahmad SA, Salih BK, Hama Hussein KF, Mikael TM, Kakamad FH, Salih AM. Aseptic meningoencephalitis after COVID-19 vaccination: a case report. Ann Med Surg (Lond) 71: 103028, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Saito K, Shimizu T, Suzuki-Inoue K, Ishida T, Wada Y. Aseptic meningitis after vaccination of the BNT162b2 mRNA COVID-19 vaccine. Neurol Sci 42: 4433-4435, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reis Carneiro D, Matos A, Morgadinho A. Steroid-responsive aseptic meningitis after BNT162b2 SARS-CoV-2 vaccine. Rev Neurol (Paris) 178: 160-161, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chan AC, Tan BY, Goh Y, Tan SS, Tambyah PA. Aseptic meningitis after BNT-162b2 COVID-19 vaccination. Brain Behav Immun Health 19: 100406, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen CJ, Hsieh LL, Lin SK, et al. Optimization of the CDC protocol of molecular diagnosis of COVID-19 for timely diagnosis. Diagnostics (Basel) 10: 333-338, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Venkatesan A, Tunkel AR, Bloch KC, et al. ; International Encephalitis Consortium. Case definitions, diagnostic algorithms, and priorities in encephalitis: consensus statement of the international encephalitis consortium. Clin Infect Dis 57: 1114-1128, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rodriguez Alvarez M, Rodríguez Valencia LM, Seidman R, et al. Rheumatoid meningitis and infection in absence of rheumatoid arthritis history: review of 31 cases. Clin Rheumatol 39: 3833-3845, 2020. [DOI] [PubMed] [Google Scholar]
- 13. Inoue C, Hagiya H, Nishimura Y, Sui O. Rheumatoid meningoencephalitis. Intern Med 59: 3255-3256, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Higashida-Konishi M, Izumi K, Tsukamoto M, et al. Anti-cyclic citrullinated peptide antibody in the cerebrospinal fluid in patients with rheumatoid arthritis who have central nervous system involvement. Clin Rheumatol 39: 2441-2448, 2020. [DOI] [PubMed] [Google Scholar]
- 15. Mondal R, Ganguly U, Deb S, et al. Meningoencephalitis associated with COVID-19: a systematic review. J Neurovirol 27: 12-25, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lv P, Peng F, Zhang Y, et al. COVID-19-associated meningoencephalitis: a care report and literature review. Exp Ther Med 21: 362-368, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lamb YN. BNT162b2 mRNA COVID-19 vaccine: first approval. Drugs 8: 495-501, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lubomski M, Sy J, Buckland M, et al. Rheumatoid leptomeningitis presenting with an acute neuropsychiatric disorder. Pract Neurol 19: 68-71, 2019. [DOI] [PubMed] [Google Scholar]
- 19. Lattanzi S, Cagnetti C, Di Bella P, et al. Leptomeningeal inflammation in rheumatoid arthritis. Neurol Neuroimmunol Neuroinflamm 1: e43, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Reindl M, Waters P. Myelin oligodendrocyte glycoprotein antibodies in neurological disease. Nat Rev Neurol 15: 89-102, 2019. [DOI] [PubMed] [Google Scholar]