Figure 3. WEE1/CDC2 physically interact with the IKK/RELA complex in a WEE1 kinase dependent manner.
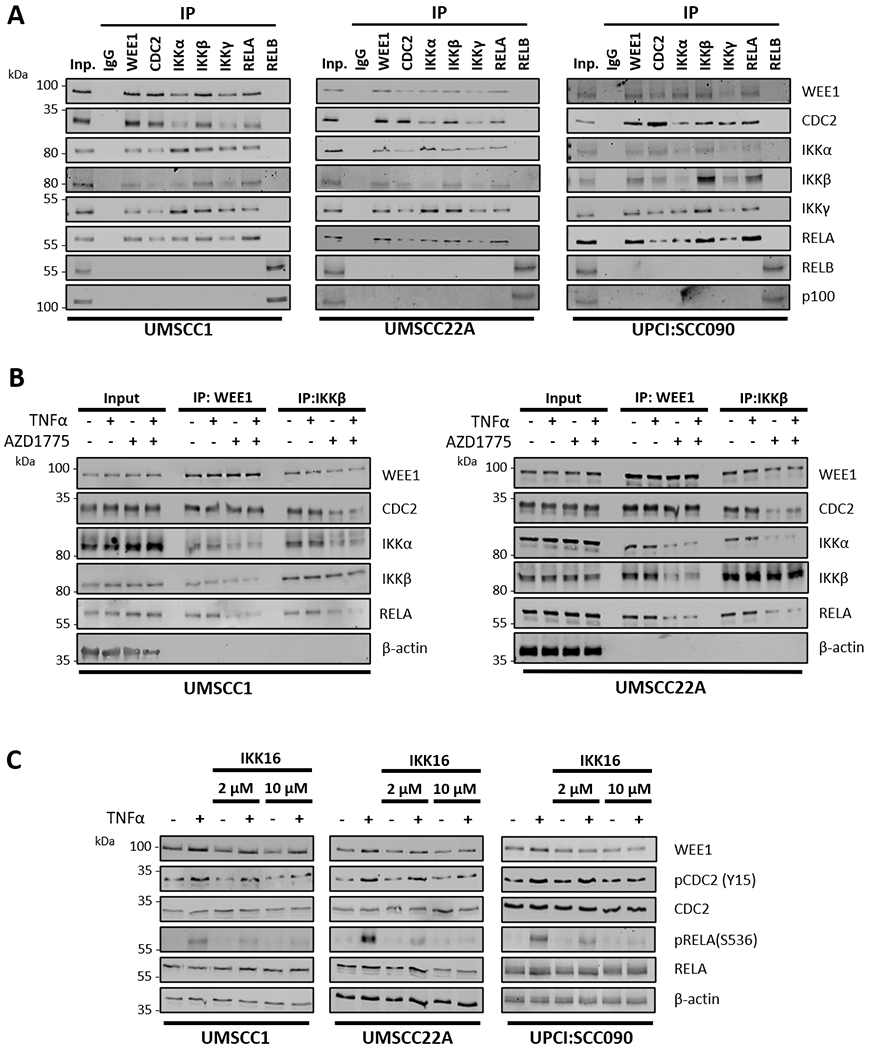
(A) Endogenous WEE1, CDC2, IKKα, IKKβ, IKKγ, RELA and RELB were immunoprecipitated from HPV− UMSCC1, HPV− UMSCC22A and HPV+ UPCI:SCC090 cells using the corresponding antibodies. Co-immunoprecipitated proteins were detected by western blot using the respective antibodies. p100 was detected as a positive control for the RELB IP. IgG was used as a specificity control. (B) UMSCC1 and UMSCC22A cells were treated with AZD1775 for six hours. 30 minutes before harvest, 10 ng/ml TNFα was added. Endogenous WEE1 and IKKβ were immunoprecipitated from UMSCC1 and UMSCC22A using the corresponding antibodies. Co-immunoprecipitated proteins were detected by western blot using the respective antibodies. β-actin was used as the loading control. (C) UMSCC1, UMSCC22A, UPCI:SCC090 cells were treated with 2 μM and 10 μM IKK16 for 6 hours. 30 minutes before harvest, 10 ng/ml TNFα was added. Whole cell lysates were analyzed by western blot for the expression of WEE1 and total and phosphorylated of CDC2, IKKα/β, and RELA. β-actin was used as the loading control.
