Abstract
In the present study, we examined associations between circadian preference and psychiatric symptoms among 1,796 pregnant from Lima, Peru. One quarter were classified as evening types. Compared to morning types, evening type pregnant women had increased odds of generalized anxiety (OR= 1.44; 95%CI: 1.12–1.86) and posttraumatic stress disorder (OR= 1.38; 95%CI: 1.07–1.78). Although there was a positive trend, evening chronotype was not significantly associated with elevated odds of depression (OR= 1.23; 95%CI: 0.94–1.61). Future studies are warranted to help understand the underlying behavioral, biological, and genetic pathways of these associations. Assessing circadian preference may help clinicians identify pregnant women at risk for psychiatric symptoms.
Keywords: circadian rhythm, depression, anxiety, posttraumatic stress disorder, pregnancy
Introduction
Circadian dysregulation has been implicated in the pathogenesis of mood and other psychiatric disorders [1–3]. Notably, evening-types are more likely to report symptoms of psychiatric disorders [1], including depression [3, 4], anxiety [5, 6], bipolar disorder [7, 8], and posttraumatic stress disorder (PTSD) [9]. The literature concerning circadian preference and psychiatric disorders, however, is quite sparse for pregnant women, a population known to be particularly vulnerable to profound sleep disturbances [10–14] and psychiatric disorders during gestation and the postpartum period [10, 15–17]. As psychological distress during pregnancy may increase the risk of adverse maternal and infant health outcomes, we sought to examine the association of circadian preference with symptoms of depression, anxiety, and PTSD in a cohort of well-characterized pregnant women enrolled in a prospective cohort study [18, 19].
Methods
Study population
This cross-sectional study is part of the Pregnancy Outcomes, Maternal and Infant Cohort Study (PrOMIS) conducted at the Instituto Nacional Materno Perinatal (INMP) in Lima, Peru between February 2012 and July 2014. Details of the study have been described previously [18, 20]. Briefly, PrOMIS was a longitudinal cohort study that examined social and behavioral maternal risk factors of pregnancy outcomes. Participants were pregnant women aged 18–47 who had their first prenatal care visit at the INMP. Women were eligible if they spoke and read Spanish, initiated prenatal care before 16 weeks’ gestation, and planned to deliver at INMP. Women were excluded from the study if they were younger than 18 years of age or had completed more than 18 weeks’ gestation. Though multiple interviews were conducted during the cohort, only information from the first interview was used for this study, which occurred during the first prenatal care visit (mean gestational age of 11 weeks). Participants were interviewed about sociodemographic and lifestyle characteristics, medical and reproductive histories, abuse history, and chronotype characteristics. All participants provided written informed consent and institutional review boards of the INMP, Lima, Peru, and the Harvard T.H. Chan School of Public Health, Office of Human Research Administration, Boston, MA, approved all procedures used in the study.
Munich Chronotype Questionnaire (MCTQ)
The Spanish-language Munich Chronotype Questionnaire was used to assess the sleep corrected mid-sleep on free days (MSFsc) [21]. The MSFsc assesses a participants’ chronotype based on local time of mid-sleep on free days corrected for sleep debt accumulated during the workweek, excluding those who use alarm clocks or don’t wake voluntarily free of disturbances. The MSFsc was calculated using the formula
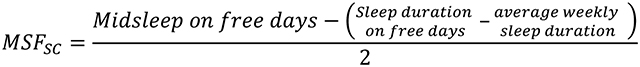 |
The MSFsc calculation is consistent with what was done in previous studies [22, 23]. The MSFsc scores range between 0 and 12 with lower values corresponding to morningness and higher values corresponding to eveningness. Given the lack of established cutoffs, scores were binned into three tertiles. A total of 44.3% of women were in tertile 1 (morning), 31.3% tertile 2 (intermediate), and 24.6% tertile 3 (evening type).
Anxiety Symptoms (GAD-7)
Symptoms of generalized anxiety were assessed using a Spanish-language version of the Generalized Anxiety Disorder-7 Questionnaire (GAD-7) [24, 25]. The GAD-7 is a 7-item questionnaire that examines the following symptoms of generalized anxiety in the two-week period prior to screening: 1) nervousness; 2) inability to stop worrying; 3) excessive worry; 4) restlessness; 5) difficulty in relaxing; 6) easily irritated; and 7) fear of something awful happening. Participants were asked how often they were bothered by each of these symptoms with response categories of: “not at all,” “several days,” “more than half the days,” and “nearly every day,” scored from 0–3 for a total score of 0 to 21. A validation study performed on our cohort of Spanish-speaking pregnant women found the GAD-7 to have good reliability and validity. Based on this study, generalized anxiety was defined as a GAD total score ≥7 (sensitivity=73.3%; specificity=67.3%) [26]. The Cronbach’s alpha, a measure of internal consistency, in the present study is 0.84.
PTSD Symptoms (PCL-C)
A Spanish-language version of the PTSD Checklist–Civilian (PCL-C) questionnaire was used to assess the symptomology and severity of PTSD during the month preceding the interview [27, 28]. The PCL-C consists of 17 items that assess three symptom clusters: re-experiencing the trauma, avoidance and numbing, and hyperarousal. Responses were scored on a 5-point Likert scale from “not at all bothersome” to “extremely bothersome.” Total PCL-C score ranges from 17 to 85, with higher scores indicating a higher severity of PTSD symptoms. A PCL-C score ≥ 26 was used to indicate presence of PTSD symptoms in our population [29]. The PCL-C has been validated in our cohort of Spanish-speaking pregnant women [29]. The Cronbach’s alpha in the present study is 0.88.
Depressive Symptoms (PHQ-9)
Symptoms of antepartum depression were assessed using the Spanish-language Patient Health Questionnaire-9 (PHQ-9) [30]. The PHQ-9 has been validated among Spanish-speaking pregnant populations [31, 32]. The PHQ-9 asks about depressive symptoms in the two weeks prior and assigns scores of 0–3 to the following responses: ‘never,’ ‘several days,’ ‘more than half the days,’ and ‘nearly every day.’ As a severity measure, the total score of PHQ-9 scores range from 0 to 27. Depression was defined as a score ≥ 10 [33]. The PHQ-9 has been validated in our cohort of Spanish-speaking pregnant women [31, 32] and has been found to have good reliability [34]. The Cronbach’s alpha in the present study is 0.76.
Additional Covariates
Sociodemographic and pregnancy variables included were categorized as follows: age (18–19, 20–29, 30–34, and ≥ 35 years); maternal race (Mestizo vs. others); years of education (≤6, 7–12, and >12 years); marital status (married/living with partner vs. others); employment status (yes vs. no); difficulty accessing basic foods (hard vs. not very hard); parity (nulliparous vs. multiparous); planned current pregnancy (yes vs. no); smoke during pregnancy (yes vs. no); alcohol consumption before pregnancy (yes vs. no); gestational age at interview (in weeks); early pregnancy body mass index (BMI): underweight (<18.5 kg/m2), normal (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2), obese (≥30 kg/m2); and self-reported health in the last year (good vs. poor).
Statistical analysis
Multivariable logistic regression procedures were performed with chronotype as the main predictor variable and psychiatric symptoms (antepartum depression, generalized anxiety, PTSD) as the outcome variable. Potential a priori confounders were included in the models. Morning chronotype was used as the reference group. Statistical analyses were performed using IBM SPSS Statistics 21.0 (SPSS Inc, Chicago, Illinois).
Results
1796 participants were included in the analysis. Mean gestational age was 11 weeks. The majority of participants were of Mestizo ethnicity [mixed European and Indigenous ancestry] (80.0%), unemployed (52.1%), and had >12 years of education (55.0%). Difficulty of getting basics including foods was reported by 43.9% of participants. A total of 31.2% of women experienced symptoms of generalized anxiety, 30.6% experienced symptoms of PTSD, and 24.9% experienced symptoms of antepartum depression (See Table 1).
Table 1:
Characteristics of population according to circadian preference in PrOMIS 2 (N = 1,796)
| Characteristics | All participants (N = 1,796) | Morning type: (N= 796) | Intermediate type: (N= 558) | Evening type: (N= 442) | p-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | ||
| Age (years)a | 27.77 ± 6.25 | 28.76 ± 6.43 | 27.86 ± 6.12 | 25.86 ± 5.65 | <0.001 | ||||
| Age (years) | |||||||||
| 18–20 | 118 | 6.6 | 46 | 5.8 | 32 | 5.7 | 40 | 9.0 | <0.001 |
| 20–29 | 1010 | 56.2 | 406 | 51.0 | 319 | 57.2 | 285 | 64.5 | |
| 30–34 | 377 | 21.0 | 177 | 22.2 | 120 | 21.5 | 80 | 18.1 | |
| ≥35 | 291 | 16.2 | 167 | 21.0 | 87 | 15.6 | 37 | 8.4 | |
| Early pregnancy BMI (kg/m2)a | 25.68 ± 4.18 | 25.90 ± 4.32 | 25.50 ± 4.11 | 25.49 ± 3.99 | 0.155 | ||||
| Early pregnancy BMI (kg/m2) | |||||||||
| <18.5 | 36 | 2.0 | 15 | 1.9 | 12 | 2.2 | 9 | 2.0 | 0.394 |
| 18.5–24.9 | 818 | 45.5 | 347 | 43.6 | 256 | 45.9 | 215 | 48.6 | |
| 25–29.9 | 676 | 37.6 | 298 | 37.4 | 217 | 38.9 | 161 | 36.4 | |
| ≥30 | 248 | 13.8 | 125 | 15.7 | 68 | 12.2 | 55 | 12.4 | |
| Gestational age at interviewa | 11.17 ± 3.93 | 11.03 ± 3.91 | 11.30 ± 3.96 | 11.27 ± 3.95 | 0.341 | ||||
| Education (years) | |||||||||
| ≤6 | 22 | 1.2 | 12 | 1.5 | 6 | 1.1 | 4 | 0.9 | 0.007 |
| 7–12 | 779 | 43.4 | 380 | 47.7 | 231 | 41.4 | 168 | 38.0 | |
| >12 | 987 | 55.0 | 402 | 50.5 | 316 | 56.6 | 269 | 60.9 | |
| Mestizo ethnicity | 1437 | 80.0 | 645 | 81.0 | 440 | 78.9 | 352 | 79.6 | 0.781 |
| Married/living with a partner | 1479 | 82.4 | 657 | 82.5 | 474 | 85.3 | 348 | 78.7 | 0.027 |
| Employed | 861 | 47.9 | 421 | 52.9 | 249 | 44.6 | 191 | 43.2 | 0.001 |
| Difficulty accessing basic food | 786 | 43.9 | 351 | 44.3 | 261 | 47.0 | 174 | 39.4 | 0.051 |
| Difficulty accessing medical care | 740 | 41.5 | 347 | 43.9 | 224 | 40.8 | 169 | 38.3 | 0.152 |
| Nulliparous | 836 | 46.6 | 342 | 43.0 | 247 | 44.3 | 247 | 55.9 | <0.001 |
| Planned pregnancy | 690 | 38.4 | 303 | 38.1 | 218 | 39.1 | 169 | 38.2 | 0.800 |
| Tobacco smoking before pregnancy | 265 | 14.8 | 89 | 11.2 | 75 | 13.4 | 101 | 22.9 | <0.001 |
| Tobacco smoking during pregnancy | 33 | 1.8 | 13 | 1.6 | 5 | 0.9 | 15 | 3.4 | 0.026 |
| Alcohol consumption before pregnancy | 574 | 32.0 | 211 | 26.5 | 181 | 32.4 | 182 | 41.2 | <0.001 |
| Alcohol consumption during pregnancy | 93 | 5.2 | 29 | 3.6 | 32 | 5.7 | 32 | 7.2 | 0.053 |
| Antepartum generalized anxietya | 5.15 ± 3.84 | 4.92 ± 3.81 | 5.00 ± 3.59 | 5.75 ± 4.14 | 0.001 | ||||
| Antepartum generalized anxiety (GAD ≥ 7) | 560 | 31.2 | 230 | 28.9 | 174 | 31.2 | 156 | 35.3 | 0.066 |
| Antepartum PTSDa | 23.63 ± 7.69 | 23.53 ± 7.52 | 23.15 ± 7.06 | 24.41 ± 8.64 | 0.035 | ||||
| Antepartum PTSD (PCL ≥ 26) | 550 | 30.6 | 228 | 28.6 | 166 | 29.7 | 156 | 35.3 | 0.045 |
| Antepartum depressiona | 6.92 ± 4.36 | 6.69 ± 4.26 | 6.92 ± 4.25 | 7.34 ± 4.65 | 0.037 | ||||
| Antepartum depression (PHQ-9 ≥ 10) | 448 | 24.9 | 191 | 24.0 | 138 | 24.7 | 119 | 26.9 | 0.517 |
Due to missing data, percentages may not add up to 100%. For continuous variables, P-value was calculated using ANOVA; for categorical values, P-value was calculated using Chi-square test. Abbreviations: BMI; Body Mass Index.
Mean ± standard deviation
Among participants, the overall mean MSFsc was 2.67 ±1.38 (range=0–23). The mean MSFsc for the morning types was 1.50 (SD=0.69), for intermediate types 3.0 (SD=0), and for evening types 4.36 (SD=1.17). Compared to morning types, evening types had increased odds of experiencing symptoms of generalized anxiety (OR=1.44; 95%CI:1.12–1.86) and PTSD (OR=1.38; 95%CI:1.07–1.78). However, evening types did not have significantly increased odds of experiencing symptoms of depression (OR=1.23; 95%CI:0.94–1.61) (See Table 2).
Table 2.
Association of circadian preference with psychiatric symptoms during pregnancy
| No psychiatric symptoms | Psychiatric symptoms | |||||
|---|---|---|---|---|---|---|
| N | % | N | % | Unadjusted OR (95% CI) | Adjusted OR (95% CI)a | |
| Antepartum generalized anxiety | ||||||
| Circadian preference | ||||||
| Morning | 566 | 45.8 | 230 | 41.1 | Reference | Reference |
| Intermediate | 384 | 31.1 | 174 | 31.1 | 1.12 (0.88–1.41) | 1.14 (0.90–1.45) |
| Evening | 286 | 23.1 | 156 | 27.9 | 1.34 (1.05–1.72) | 1.44 (1.12–1.86) |
| p-value for trend | 0.022 | 0.005 | ||||
| Antepartum PTSD | ||||||
| Circadian preference | ||||||
| Morning | 568 | 45.6 | 228 | 41.5 | Reference | Reference |
| Intermediate | 392 | 31.5 | 166 | 30.2 | 1.06 (1.83–1.34) | 1.04 (0.82–1.32) |
| Evening | 286 | 23.0 | 156 | 28.4 | 1.36 (1.06–1.74) | 1.38 (1.07–1.78) |
| p-value for trend | 0.021 | 0.014 | ||||
| Antepartum depression | ||||||
| Circadian preference | ||||||
| Morning | 605 | 44.9 | 191 | 42.6 | Reference | Reference |
| Intermediate | 420 | 31.2 | 138 | 30.8 | 1.04 (0.81–1.34) | 1.04 (0.81–1.35) |
| Evening | 323 | 24.0 | 119 | 26.6 | 1.17 (0.90–1.52) | 1.23 (0.94–1.61) |
| p-value for trend | 0.270 | 0.145 | ||||
Abbreviations: OR, odds ratio; CI, confidence interval.
Adjusted for age (continuous), marital status (married vs. others), parity (nulliparous vs. others), difficulty paying for the very basics (hard vs. not very hard)
Discussion
Evening type pregnant women were more likely to experience symptoms of generalized anxiety and PTSD compared to morning types, while intermediate types did not have elevated odds of psychiatric disturbances. Morningness-eveningness was not associated with symptoms of depression.
While no previous studies have examined the association between morningness-eveningness and anxiety during pregnancy, this association has been studied among non-pregnant populations. Our findings are consistent with most [6, 35, 36] though not all [5, 23] prior studies that reported a positive association between eveningness and anxiety. We are aware of one previous study that examined the association between chronotype and PTSD. Hasler et al in their study among combat veterans [9] found a positive correlation between evening chronotype and PTSD (r=0.41). This is not surprising as one of the hallmarks of PTSD is a state of hyperarousal which is characterized by difficulty initiating and maintaining sleep [9].
While we found one study demonstrating higher odds of depression among morning types [35], the majority of previous studies have largely shown that evening types are more likely to report symptoms of depression [3, 5, 37–41]. Although a positive trend was present, we did not find a significant association between chronotype and depression in our population of pregnant women. There are a few possible explanations for our findings. Other studies show participants with depression are more likely to be intermediate than morning or evening types [42–44]. Furthermore, the prevalence of depression has been found to increase later in pregnancy [45] and the post-partum period [46], and this study assessed women at an average gestational age of 11 weeks. Another possible explanation is that sleep disturbances are common during pregnancy, which may alter the relationship between chronotype on mood [12, 47, 48].
Previous studies have demonstrated a connection between morningness-eveningness and psychological health. Mood disorders, including depression and bipolar disorder, are associated with circadian rhythm disruptions affecting sleep cycles, body temperature regulation, blood pressure, and secretion of cortisol and melatonin [49, 50]. Furthermore, reproductive hormones may alter rhythmicity and contribute to mood disturbances during pregnancy [51]. There is growing evidence that chronotypes are associated with the immune system and maintenance of biological systems during pregnancy [52]. Understanding chronotype disturbances during pregnancy is important given the association between psychiatric disorders and poor infant neurocognitive and physical development outcomes.
Our study expands the literature by assessing the association between morningness-eveningness and psychiatric symptoms among pregnant Peruvian women. The strengths of our study include a large sample size and use of previously validated questionnaires. However, our study has some limitations. First, the study was conducted at a single hospital in Lima, Peru, which primarily serves a low-income population, and our results may not be generalizable to other pregnant populations. Second, generalized anxiety, PTSD, and depression were assessed based on screening instruments. Although the use of brief screening instruments is the most feasible method of data collection for large-scale epidemiologic studies, future studies using diagnostic evaluations for mood disorders are warranted.
Overall, our findings suggest that circadian preference has important implications for psychiatric disorders during pregnancy. When compared to morning types, evening type pregnant women have significantly increased odds of experiencing generalized anxiety and PTSD symptoms. As the prevalence and severity of psychiatric disorders vary during pregnancy, longitudinal studies are warranted to help understand how circadian preference throughout pregnancy may impact psychiatric symptoms. Assessing morningness-eveningness may help clinicians identify pregnant women at increased risk of experiencing symptoms of anxiety and PTSD during pregnancy and may also contribute to our understanding of the impact of circadian rhythm on the overall health of pregnant women.
Acknowledgments
The authors wish to thank the dedicated staff members of Asociación Civil Proyectos en Salud (PROESA), Perú and Instituto Materno Perinatal, Perú for their expert technical assistance with this research.
Funding
This research was supported by awards from the National Institutes of Health (NIH), National Institute of Minority Health and Health Disparities (T37-MD-001449) and Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01-HD-059835; R01HD102342).
Footnotes
Declaration of interest statement
All authors declare no conflicts of interest.
Data availability statement
Data is available upon request.
References
- 1.Rose D, Gelaye B, Sanchez S, Castaneda B, Sanchez E, Yanez ND, Williams MA: Morningness/eveningness chronotype, poor sleep quality, and daytime sleepiness in relation to common mental disorders among Peruvian college students. Psychol Health Med 2015, 20(3):345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mendoza J: Circadian insights into the biology of depression: Symptoms, treatments and animal models. Behav Brain Res 2019, 376:112186. [DOI] [PubMed] [Google Scholar]
- 3.Merikanto I, Lahti T, Kronholm E, Peltonen M, Laatikainen T, Vartiainen E, Salomaa V, Partonen T: Evening types are prone to depression. Chronobiol Int 2013, 30(5):719–725. [DOI] [PubMed] [Google Scholar]
- 4.Au J, Reece J: The relationship between chronotype and depressive symptoms: A meta-analysis. J Affect Disord 2017, 218:93–104. [DOI] [PubMed] [Google Scholar]
- 5.Antypa N, Vogelzangs N, Meesters Y, Schoevers R, Penninx BW: Chronotype Associations with Depression and Anxiety Disorders in a Large Cohort Study. Depress Anxiety 2016, 33(1):75–83. [DOI] [PubMed] [Google Scholar]
- 6.Passos GS, Santana MG, Poyares D, D’Aurea CV, Teixeira AA, Tufik S, de Mello MT: Chronotype and anxiety are associated in patients with chronic primary insomnia. Braz J Psychiatry 2017, 39(2):183–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melo MCA, Abreu RLC, Linhares Neto VB, de Bruin PFC, de Bruin VMS: Chronotype and circadian rhythm in bipolar disorder: A systematic review. Sleep Med Rev 2017, 34:46–58. [DOI] [PubMed] [Google Scholar]
- 8.Wood J, Birmaher B, Axelson D, Ehmann M, Kalas C, Monk K, Turkin S, Kupfer DJ, Brent D, Monk TH et al. : Replicable differences in preferred circadian phase between bipolar disorder patients and control individuals. Psychiatry Res 2009, 166(2–3):201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hasler BP, Insana SP, James JA, Germain A: Evening-type military veterans report worse lifetime posttraumatic stress symptoms and greater brainstem activity across wakefulness and REM sleep. Biol Psychol 2013, 94(2):255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gelaye B, Addae G, Neway B, Larrabure-Torrealva GT, Qiu C, Stoner L, Luque Fernandez MA, Sanchez SE, Williams MA: Poor sleep quality, antepartum depression and suicidal ideation among pregnant women. J Affect Disord 2017, 209:195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rice JR, Larrabure-Torrealva GT, Luque Fernandez MA, Grande M, Motta V, Barrios YV, Sanchez S, Gelaye B, Williams MA: High risk for obstructive sleep apnea and other sleep disorders among overweight and obese pregnant women. BMC Pregnancy Childbirth 2015, 15:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mindell JA, Cook RA, Nikolovski J: Sleep patterns and sleep disturbances across pregnancy. Sleep Med 2015, 16(4):483–488. [DOI] [PubMed] [Google Scholar]
- 13.Bei B, Coo S, Trinder J: Sleep and Mood During Pregnancy and the Postpartum Period. Sleep Med Clin 2015, 10(1):25–33. [DOI] [PubMed] [Google Scholar]
- 14.Sedov ID, Cameron EE, Madigan S, Tomfohr-Madsen LM: Sleep quality during pregnancy: A meta-analysis. Sleep Med Rev 2018, 38:168–176. [DOI] [PubMed] [Google Scholar]
- 15.Orta OR, Gelaye B, Qiu C, Stoner L, Williams MA: Depression, anxiety and stress among pregnant migraineurs in a pacific-northwest cohort. J Affect Disord 2015, 172:390–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Becker M, Weinberger T, Chandy A, Schmukler S: Depression During Pregnancy and Postpartum. Curr Psychiatry Rep 2016, 18(3):32. [DOI] [PubMed] [Google Scholar]
- 17.Fairbrother N, Young AH, Janssen P, Antony MM, Tucker E: Depression and anxiety during the perinatal period. BMC Psychiatry 2015, 15:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barrios YV, Gelaye B, Zhong Q, Nicolaidis C, Rondon MB, Garcia PJ, Sanchez PA, Sanchez SE, Williams MA: Association of childhood physical and sexual abuse with intimate partner violence, poor general health and depressive symptoms among pregnant women. PLoS One 2015, 10(1):e0116609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhong QY, Wells A, Rondon MB, Williams MA, Barrios YV, Sanchez SE, Gelaye B: Childhood abuse and suicidal ideation in a cohort of pregnant Peruvian women. Am J Obstet Gynecol 2016, 215(4):501 e501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedman LE, Gelaye B, Rondon MB, Sanchez SE, Peterlin BL, Williams MA: Association of migraine headaches with suicidal ideation among pregnant women in Lima, Peru. Headache 2016, 56(4):741–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roenneberg T, Wirz-Justice A, Merrow M: Life between clocks: daily temporal patterns of human chronotypes. J Biol Rhythms 2003, 18(1):80–90. [DOI] [PubMed] [Google Scholar]
- 22.Felden ÉPG, Filipin D, Barbosa DG, Andrade RD, Meyer C, Louzada FM: Factors associated with short sleep duration in adolescents. Revista Paulista de Pediatria (English Edition) 2016, 34(1):64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kantermann T, Theadom A, Roenneberg T, Cropley M: Fibromyalgia syndrome and chronotype: late chronotypes are more affected. J Biol Rhythms 2012, 27(2):176–179. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Campayo J, Zamorano E, Ruiz MA, Pardo A, Perez-Paramo M, Lopez-Gomez V, Freire O, Rejas J: Cultural adaptation into Spanish of the generalized anxiety disorder-7 (GAD-7) scale as a screening tool. Health and Quality of Life Outcomes 2010, 8(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spitzer R, Kroenke K, Williams J, Löwe B: A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006, 166(10):1092–1097. [DOI] [PubMed] [Google Scholar]
- 26.Zhong QY, Gelaye B, Zaslavsky AM, Fann JR, Rondon MB, Sanchez SE, Williams MA: Diagnostic Validity of the Generalized Anxiety Disorder - 7 (GAD-7) among Pregnant Women. PLoS One 2015, 10(4):e0125096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conybeare D, Behar E, Solomon A, Newman MG, Borkovec TD: The PTSD Checklist-Civilian Version: reliability, validity, and factor structure in a nonclinical sample. Journal of Clinical Psychology 2012, 68(6):699–713. [DOI] [PubMed] [Google Scholar]
- 28.Weathers F, Huska J, Keane T: PCL-C for DSM-IV. In. Edited by Division NCfP-BS. Boston; 1991. [Google Scholar]
- 29.Gelaye B, Zheng Y, Medina-Mora ME, Rondon MB, Sanchez SE, Williams MA: Validity of the posttraumatic stress disorders (PTSD) checklist in pregnant women. BMC Psychiatry 2017, 17(1):179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spitzer R, Kroenke K, Williams J: Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA 1999, 282(18):1737–1744. [DOI] [PubMed] [Google Scholar]
- 31.Wulsin L, Somoza E, Heck J: The feasibility of using the Spanish PHQ-9 to screen for depression in primary care in Honduras. Primary Care Companion to the Journal of Clinical Psychiatry 2002, 4(5):191–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhong Q, Gelaye B, Fann JR, Sanchez SE, Williams MA: Cross-cultural validity of the Spanish version of PHQ-9 among pregnant Peruvian women: a Rasch item response theory analysis. Journal of Affective Disorders 2014, 158:148–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kroenke K, Spitzer RL, Williams JB: The Phq‐9. J Gen Intern Med 2001, 16(9):606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhong Q, Gelaye B, Rondon M, Sánchez SE, García PJ, Sánchez E, Barrios YV, Simon GE, Henderson DC, Cripe S May et al. : Comparative performance of Patient Health Questionnaire-9 and Edinburgh Postnatal Depression Scale for screening antepartum depression. Journal of Affective Disorders 2014, 162:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lemoine P, Zawieja P, Ohayon MM: Associations between morningness/eveningness and psychopathology: an epidemiological survey in three in-patient psychiatric clinics. J Psychiatr Res 2013, 47(8):1095–1098. [DOI] [PubMed] [Google Scholar]
- 36.Park CI, An SK, Kim HW, Koh MJ, Namkoong K, Kang JI, Kim SJ: Relationships between chronotypes and affective temperaments in healthy young adults. J Affect Disord 2015, 175:256–259. [DOI] [PubMed] [Google Scholar]
- 37.Yun JA, Ahn YS, Jeong KS, Joo EJ, Choi KS: The Relationship between Chronotype and Sleep Quality in Korean Firefighters. Clin Psychopharmacol Neurosci 2015, 13(2):201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hidalgo MP, Caumo W, Posser M, Coccaro SB, Camozzato AL, Chaves ML: Relationship between depressive mood and chronotype in healthy subjects. Psychiatry Clin Neurosci 2009, 63(3):283–290. [DOI] [PubMed] [Google Scholar]
- 39.Hasler BP, Allen JJ, Sbarra DA, Bootzin RR, Bernert RA: Morningness-eveningness and depression: preliminary evidence for the role of the behavioral activation system and positive affect. Psychiatry Res 2010, 176(2–3):166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kitamura S, Hida A, Watanabe M, Enomoto M, Aritake-Okada S, Moriguchi Y, Kamei Y, Mishima K: Evening preference is related to the incidence of depressive states independent of sleep-wake conditions. Chronobiol Int 2010, 27(9–10):1797–1812. [DOI] [PubMed] [Google Scholar]
- 41.Levandovski R, Dantas G, Fernandes LC, Caumo W, Torres I, Roenneberg T, Hidalgo MP, Allebrandt KV: Depression scores associate with chronotype and social jetlag in a rural population. Chronobiol Int 2011, 28(9):771–778. [DOI] [PubMed] [Google Scholar]
- 42.Gaspar-Barba E, Calati R, Cruz-Fuentes CS, Ontiveros-Uribe MP, Natale V, De Ronchi D, Serretti A: Depressive symptomatology is influenced by chronotypes. J Affect Disord 2009, 119(1–3):100–106. [DOI] [PubMed] [Google Scholar]
- 43.Müller MJ, Cabanel N, Olschinski C, Jochim D, Kundermann B: Chronotypes in patients with nonseasonal depressive disorder: Distribution, stability and association with clinical variables. Chronobiology International 2015, 32(10):1343–1351. [DOI] [PubMed] [Google Scholar]
- 44.Chan JW, Lam SP, Li SX, Yu MW, Chan NY, Zhang J, Wing YK: Eveningness and insomnia: independent risk factors of nonremission in major depressive disorder. Sleep 2014, 37(5):911–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Truijens SEM, Spek V, van Son MJM, Guid Oei S, Pop VJM: Different patterns of depressive symptoms during pregnancy. Arch Womens Ment Health 2017, 20(4):539–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Obeysekare JL, Cohen ZL, Coles ME, Pearlstein TB, Monzon C, Flynn EE, Sharkey KM: Delayed sleep timing and circadian rhythms in pregnancy and transdiagnostic symptoms associated with postpartum depression. Transl Psychiatry 2020, 10(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Skouteris H, Germano C, Wertheim EH, Paxton SJ, Milgrom J: Sleep quality and depression during pregnancy: a prospective study. J Sleep Res 2008, 17(2):217–220. [DOI] [PubMed] [Google Scholar]
- 48.Volkovich E, Tikotzky L, Manber R: Objective and subjective sleep during pregnancy: links with depressive and anxiety symptoms. Arch Womens Ment Health 2016, 19(1):173–181. [DOI] [PubMed] [Google Scholar]
- 49.Wirz-Justice A: Biological rhythm disturbances in mood disorders. Int Clin Psychopharmacol 2006, 21(Suppl 1):S11–15. [DOI] [PubMed] [Google Scholar]
- 50.Kim J, Jang S, Choe HK, Chung S, Son GH, Kim K: Implications of Circadian Rhythm in Dopamine and Mood Regulation. Mol Cells 2017, 40(7):450–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parry B, Newton R: Chronobiological basis of female-specific mood disorders. Neuropsychopharmacology 2001, 25(5 Suppl):S102–108. [DOI] [PubMed] [Google Scholar]
- 52.Man GCW, Zhang T, Chen X, Wang J, Wu F, Liu Y, Wang CC, Cheong Y, Li TC: The regulations and role of circadian clock and melatonin in uterine receptivity and pregnancy-An immunological perspective. Am J Reprod Immunol 2017, 78(2). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available upon request.


