Figure 7. Adoptive cell therapy with alloprimed CXCR5+CD8+ T cells significantly enhances allograft survival in CCR5 KO kidney transplant recipients.
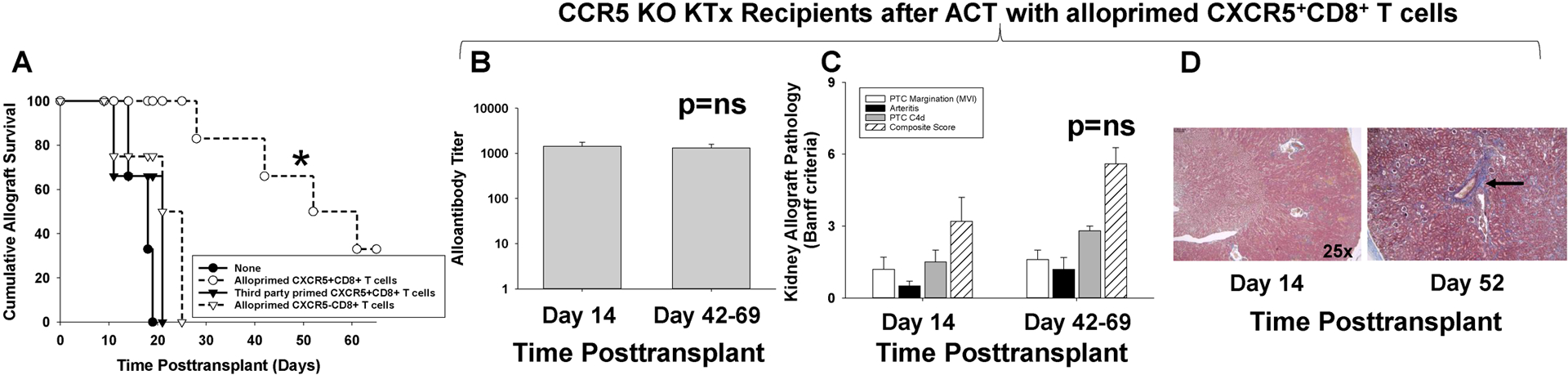
CCR5 KO (H-2b) mice were transplanted with allogeneic (A/J, H-2a) kidneys and underwent concurrent bilateral native nephrectomy. A cohort of CCR5 KO recipients received adoptive cell therapy (ACT) with 2×106 alloprimed CXCR5+CD8+ T cells (day 5 posttransplant). A) Recipients were monitored for serum creatinine (SCr) to determine allograft survival (SCr≥100 μmol/L indicates graft loss). Kidney allograft survival was significantly prolonged in recipients that received ACT with CXCR5+CD8+ T cells (MST= 52 days, n=6) as compared to CCR5 KO recipient without ACT (MST= 15 days, n=6; *p=0.0007). ACT with 2×106 third party-primed CXCR5+CD8+ T cells (MST=21, n=3) or 2×106 alloprimed CXCR5−CD8+ T cells (MST=25, n=4) into CCR5 KO kidney transplant recipients did not prolong allograft survival (p=ns for both). B-D) CCR5 KO recipients that received ACT with alloprimed CXCR5+CD8+ T cells were evaluated for alloantibody and pathology at day 14 and at time of late allograft loss. B) In CCR5 KO recipients that received ACT with alloprimed CXCR5+CD8+ T cells, alloantibody titer on day 14 (1,400±330, n=5) was similar to alloantibody titer at the time of late allograft loss (day 42–69; 1,300±260, p=ns). C) Composite AMR scores were not significantly different between kidney allografts analyzed on day 14 (3.2±1.0) or at the time of late allograft loss (5.6±1.5; p=ns). D) Trichrome staining demonstrated mild fibrosis (1.0±0.0) at the time of late allograft loss compared to day 14 kidney allograft specimens that had no evidence of fibrosis (0±0; p<0.0001). Images are representative of the sample groups. Black arrow indicates area of perivascular fibrosis.
