Figure 1.
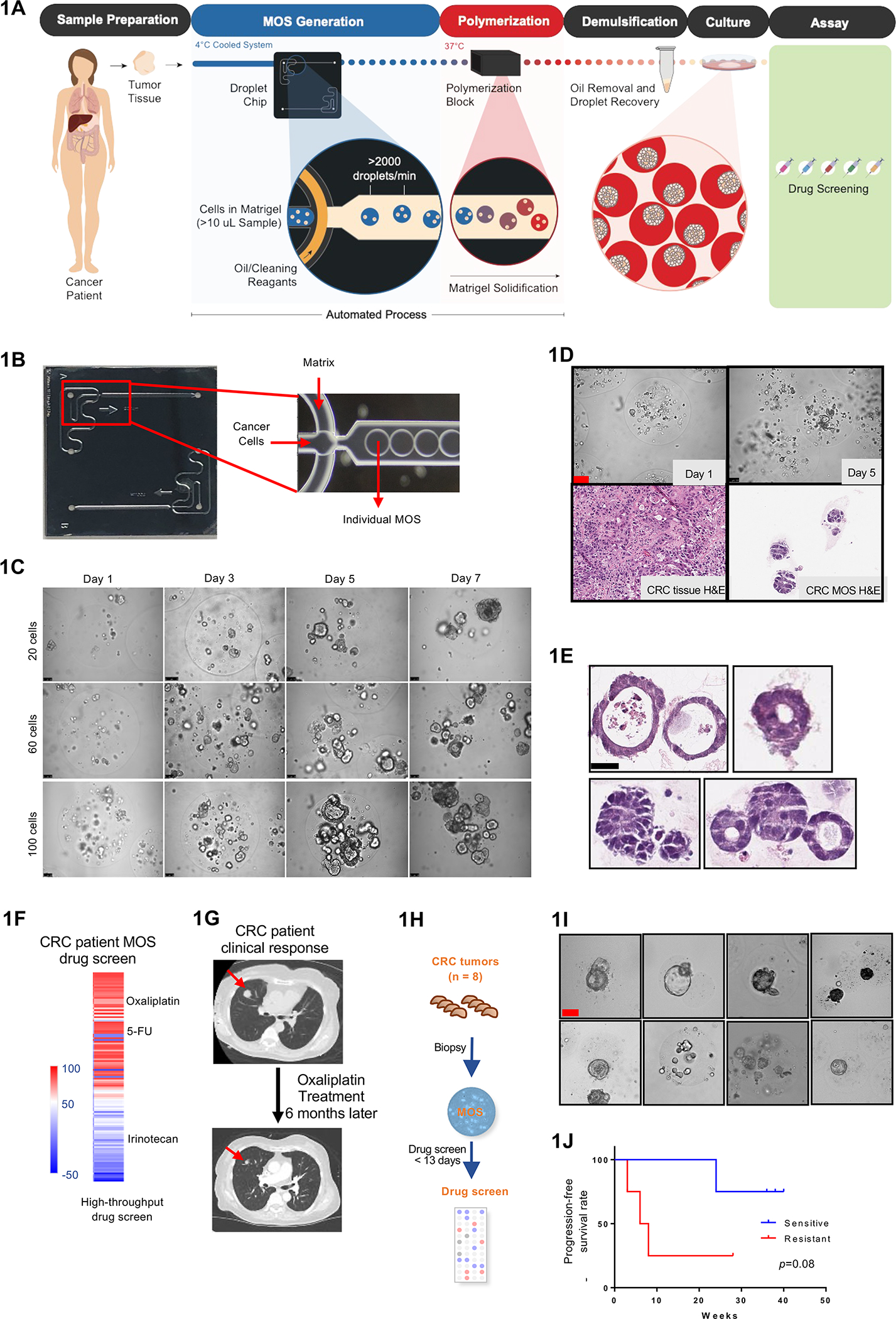
Establishing CRC MOS for drug screen and clinical validation. (A) Scheme of CRC MOS generation and drug screening. (B) Images of the microfluidic MOS chip. (C) Bright field microscope images of CRC MOS generated with different cell numbers per MOS. (D) Representative images of generated MOS from patient CRC tumor tissue and hematoxylin and eosin (H&E) staining of the primary CRC tumor tissue and derived MOS. (E) H&E staining of CRC MOS established from different patient tumor tissues. (F) Heat map of high throughput drug screen using CRC tumor-derived MOS indicates sensitivity to oxaliplatin and resistance to Irinotecan. (G) The same patient showed response to oxaliplatin after 6 months of treatment in clinic. (H) Schematic illustration of the clinical study design. MOS are established from CRC biopsy for drug testing. (I) Representative images of patient-derived MOS. (J) Survival outcomes from all eight CRC patients are correlated with MOS drug sensitivity. Scale bar: 100 um.
