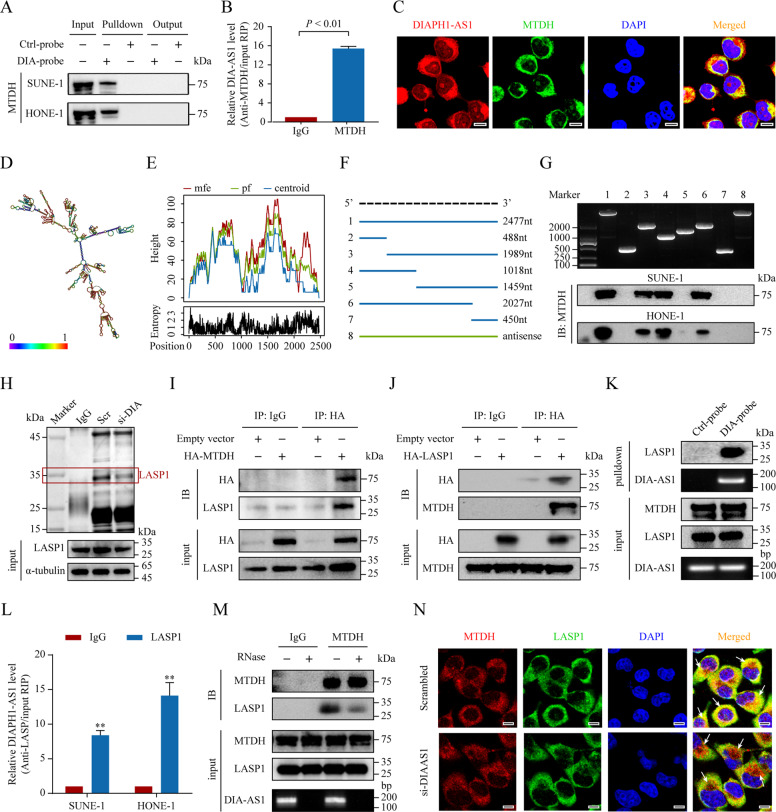
Fig. 6. DIAPH1-AS1 acts as an adapter to form MTDH-LASP1 complex.
A RNA pulldown assay showing the direct interaction of DIAPH1-AS1 with the MTDH protein. B Association of endogenous MTDH and DIAPH1-AS1 was detected using an RIP-qPCR assay. HONE-1 cell lysates were immunoprecipitated with the anti-MTDH antibodies. C FISH and IF double staining showing the subcellular co-localization of DIAPH1-AS1 and MTDH. Scale bar: 20 μm. D, E The secondary structure (D) and minimum free energy (MFE) structure (E) of DIAPH1-AS1 was analyzed using the online tool RNAfold WebServer. F, G Deletion mapping of the MTDH-binding domain in DIAPH1-AS1. F Diagrams of full-length DIAPH1-AS1 and its deletion fragments. G Top, the in vitro–transcribed full-length DIAPH1-AS1 and deletion fragments with the correct sizes are indicated. Bottom, western blotting analysis for MTDH pulled down by different DIAPH1-AS1 fragments. H An immunoprecipitation assay was performed to detect the interaction between MTDH and LASP1 in HONE-1 cells with or without DIAPH1-AS1 silencing. Isolated proteins were resolved by SDS-PAGE followed by silver staining. Differential bands were cut for mass spectrometry. I, J HONE-1 cells transfected with HA-tagged MTDH vectors (I), HA-tagged LASP1 vectors (J) or control vectors were lysed for co-IP assays using anti-HA antibodies or normal rabbit IgG. K The interaction between DIAPH1-AS1 and LASP1 was confirmed using RNA pulldown assays and western blotting analysis. L The association of endogenous LASP1 and DIAPH1-AS1 was detected using an RIP-qPCR assay. M Immunoblotting to detect of exogenous MTDH and LASP1 immunoprecipitated by anti-MTDH antibodies with or without RNase If treatment in HONE-1 cells. N The interaction between MTDH and LASP1 in HONE-1 cells, with or without DIAPH1-AS1 silencing was assessed using immunofluorescence double staining. Scale bar: 20 μm. Data are presented as the mean ± SD. *P < 0.05, **P < 0.01. The experiments were repeated at least three times independently.