Abstract
Subcutaneous nodular onchocercosis was detected and investigated in 17 Japanese Sika deer (Cervus nippon), captured in Gifu and Shiga Prefectures/Japan, in the period between 2016 and 2017. The worms were found in all the seventeen deer within characteristics subcutaneous nodules dispersed mainly in the back (especially in the lumbar region and flanks), with few scattered nodules were located at the forelimbs and neck. The all collected nodules were examined stereo-microscopically. The parasites were extracted from the nodules and identified through morphological and histopathological examinations. Molecular identification through sequencing of the following genes; internal transcribed spacer subunit 2 (ITS2)–28S ribosomal RNA (28S rRNA), cytochrome c oxidase subunit 1 (cox1) and mitochondrially encoded NADH dehydrogenase subunit 2 (NAD2) were performed. The histopathological, molecular and phylogenetic analysis demonstrated that, the filarial nematode isolated from Gifu and Shiga Prefectures in Japan is O. flexuosa. This is the first report about presence of O. flexuosa in Japanese Sika deer (Cervus nippon) in Gifu and Shiga Prefectures.
Supplementary Information
The online version supplementary material available at 10.1007/s12639-021-01453-3.
Keywords: Deer, Histopathology, Japan, Onchocerca flexuosa, Sequencing analysis
Introduction
Filaroids parasites (family Onchocercidae), mainly associated wild and domestic ungulates (e.g. cervids, horses, camelids and bovids); humans and dogs are also affected (Lefoulon et al. 2017). More than 30 species belong to the genus Onchocerca affected this domestic ungulates. Two species are found as adult worms in dogs (O. lupi) and humans (O. volvulus) (Anderson 2000; Morales-Hojas et al. 2006). These types of parasites had a worldwide distribution, in Europe and various parts of the world as Africa, America and Yemen. They are of great concern due to the disease they caused in that hosts reflecting on the general health and diminish the value of animal carcasses (Genchi et al. 2011; Otranto and Eberhard 2011; Morchón et al. 2012). These parasites resulted in characteristic lesions, according to its type, as for example; Dirofilaria. immitis infects dogs and cats, resulting in a fatal cardiocirculatory disease known as ‘heartworm disease’, D. repens is a more prevalent zoonotic pathogen in man (Dantas-Torres and Otranto 2013).
Parasitism by Onchocerca spp. in European wild Cervidae described four species of Onchocerca; O. garmsi, worms were free in subcutaneous tissue of the sternum; O. jakutensis (syn. O. tubingensis), worms creating nodules; O. skrjabini (syn. O. tarsicola), doesn’t form nodules, and adults were present in carpal and tarsal regions, while the microfilariae were found in the skin of muzzle and ears (Dantas-Torres and Otranto 2013; Bain and Schulz-Key 1974; Miller 2007), The fourth spp. O.flexuosa (Wedl, 1856), is the most prevalent species, and localized within nodules in the dorsal region and flanks, whose primary host is the European red deer (Cervus elaphus) (Bain and Schulz-Key 1974). and is easily identified by its location and morphological characters (Bain and Schulz-Key 1974), as well as by DNA sequences of the mitochondrial genes (Krueger et al. 2007).
Currently, family Cervidae are a hosts of six described Onchocerca species (Boijsen et al. 2017), these species are; O. garmsi, O. flexuosa, O. eberhardi, O. cervipedis,O. jakutensis (syn.O.tubingensis), and O. skrjabini (syn. O.tarsicola). However, in North American cervids, another undescribed species were found recently (McFrederick et al. 2013).
Routine diagnosis is mostly based upon microscopic examination to determine the morphology of the adult worm and its microfilaria. However, Giemsa staining cannot differentiate between the most related species (Bain 2002). Definitive diagnosis and control of such parasites are of great health concern, and specific molecular data will help scientists discriminate between closely related species and will also provide new drug target sites unique to the Onchocerca species.
A major strength of molecular identification depending upon specific DNA barcode, as it is allows correlation of any small part of parasites/organism to a single molecular entity (Molecular Operational Taxonomic Unit, MOTU) (Floyd et al. 2002), and taxonomy skilled personnel does not necessary required in the molecular data analysis. cox1 gene sequences and several others mitochondrial markers genes are widely used for DNA barcoding of metazoans (Markmann and Tautz 2005; Monaghan et al. 2005).
In the present study, we describe the morphological, pathological and molecular characteristics of worms of a novel Onchocerca species present inside subcutaneous nodules in the skin of a Wild Sika Deer (Cervus Nippon) in Gifu and Shiga Prefectures in Japan. The molecular diagnosis based on molecular identification of 3 genes; internal transcribed spacer subunit 2 (ITS2)–28S ribosomal RNA (28S rRNA), cytochrome C oxidase subunit 1 (cox1), and NADH dehydrogenase subunit 2 (NAD2).
Material and methods
Sample collection
“Between 2016 and 2017” 17 wild Japanese deer from Gifu and Shiga Prefectures were captured by wildlife conservation authorities. These deer presented yellowish subcutaneous nodules dispersed mainly in the back, in the sacrum region, lumbar region and flanks and rarely on the lateral abdomen or proximal parts of the extremities, in addition, few scattered nodules were located at the forelimbs and neck. The wild deers were slaughtered by licensed hunters in accordance with the policies of the Ministry of the Environment, Japan. The carcasses were refrigerated and transferred under cool conditions to the Laboratory of Veterinary Pathology, Faculty of Applied Biological Sciences, Gifu University, Japan. Five subcutaneous nodules from each deer were properly collected, cooled by ice wrapping and the collected nodules were all examined using a stereomicroscope. Parasites were detected in all the examined subcutaneous connective tissues nodules. Worms were removed with a pair of small forcipes, and saline was used for ease in detaching. The collected worms was then morphologically examined, Onchocerca nematodes were suspected to be the infective parasite based on the morphological features (Anderson et al. 2009; Uni et al. 2002). The worms collected from each nodule, rinsed in normal saline, and stored in 70% ethyl alcohol and used for parasitological and molecular identification. About 50% of the worms were used for parasitological identification and the rest of the worms were maintained at − 20 °C until used for DNA extraction and PCR analysis.
Animals used in this study were also reviewed and handled according to code of ethics of Research and Animal Resources Committee, Faculty of Applied biological science, Gifu University, Japan, approved by Gifu university animal experiment committee.
Histopathological examination
Tissue specimens of subcutaneous nodules collected from the infected deer were fixed in 10% neutral buffered formalin for histopathological examination. Fixed specimens were routinely processed through dehydration in ascending grades of ethanol, cleaned in xylene, and embedded in paraffin. Paraffin sections were obtained and stained with hematoxylin and eosin (H&E) for histopathological examinations.
DNA extraction
Total DNA extractions from worm nodules were performed using the AllPrep® DNA/RNA Mini kit and DNeasy Blood & Tissue Kit (Qiagen, Hilden Germany) according to the manufacturer’s instructions.
PCR amplification
Each DNA extract was used as a template for PCR amplification using the specific filarial primers CO1F3–CO1R3 and ND2F1–ND2R1 (Yatawara et al. 2010). In addition, parts of the nuclear ribosomal gene repeat were also amplified. The ITS2 region (with short portions of the flanking 5.8S and 28S genes) was amplified using previously published primers (Yatawara et al. 2007). The PCR technique was performed in a 50-μl volume containing 4 μl of DNA, 4 μl of dNTP, 1 μl (10 μMol) of each forward and reverse primers, 5 μl 10 × Ex Taq buffer, 0.25 μl Ex Taq polymerase (Takara, Kyoto, Japan), and 34.75 μl distilled water. The PCR cycle for NAD2 and cox1 genes was as follows: denaturation at 94 °C for 3 min; 30–35 cycles of 30 s at 94 °C; 30 s at 50 °C, and 1 min at 72 °C; followed by a final extension period of 5 min at 72 °C. PCR cycle for the ITS2 region (with short portions of the flanking 5.8S and 28S genes) was as follows: denaturation at 94 °C for 5 min; 35–40 cycles of 94 °C for 45 s, 58 °C for 45 s, and 72 °C for 90 s; followed by a final extension period at 72 °C for 7 min.
All PCRs were performed using the Takara Thermal cycler, Japan. PCR products were visualized and photographed in a 0.75%–1% agarose gel containing 0.5 mg/ml ethidium bromide on a Gel Documentation System.
DNA Sequencing, sequence alignment, and phylogenic analysis
PCR amplicons were purified and prepared for sequencing using the QIA quick gel extraction kits® according to the manufacturer’s instructions. The purified PCR products of all O. flexuosa positive samples were sequenced using the AB3500XL Genetic analyzer (Applied Biosystems, HITACHI, Japan) at the genome center of Gifu University, Japan using gene-specific primers.
The obtained nucleotide sequences of ITS2–28S rRNA, cox1, NAD2 and the deduced amino acid sequences of O. flexuosa were edited using the Sequence Scanner Software 2 program (http://www.appliedbiosystems.com). Primer sequences were omitted prior to phylogenetic analysis; the edited sequences were computationally compared with those of other filarial reference sequences for determining the homology and were subjected to phylogenetic analyses using the MEGA 7software (www.megasoftware.net/).
The phylogenetic trees were generated using the neighbor-joining (N-J) tree method, and the reliability of internal branches was assessed by 1000 bootstrap replications (Abd-Ellatieff et al. 2018; Saitou and Nei 1987). The reference sequences of filarial genes were retrieved from the GenBank database, and their accession numbers are listed in (Table. 2).
Table 1.
History of collected samples from infected deers with O. flexuosa in Gifu and Shiga Prefectures /Japan
| Place of sampling | Host age | Sex | Date | Habitat in body skin | Total | ||
| ≤ 2 years | >2 years | Male | Female | ||||
| Gifu Prefecture | 20 | 18 | 13 | 25 | 2016–2018 | Femur/Abdomen/Back/femur / All the body skin | 77 |
| Hino-Shiga Prefecture | 17 | 22 | 16 | 23 | 2016–2018 | Back/ Shoulders/ Femur/ neck skin | |
Table 2.
Sequences of Onchocerca nematode reference strains published in GenBank used for (cox1, ITS2-28S rRNA, and NAD2) genes
| Onchocerca Strain | Gene Bank Accession number | Origin |
|---|---|---|
| Onchocercaflexuosa | LC321994(I),LC318284(CO1) LC320669 (NAD2) | Japan |
| O. gibsoni isolate Ogib2c4 | DQ317647(I) | Portugal |
| O. gibsoni isolate Ogib2c5 | DQ317648(I) | Portugal |
| O. ochengiisolate Ooch3c2 | DQ317659(I) | Portugal |
| O. ochengi | FM206482(I) | Cameroon |
| O. ochengiisolate Ooch3c2 | DQ317661(I) | Portugal |
| O. volvulus isolate Ovol9c3 | DQ317664(I) | Portugal |
| O. volvulus isolate Ovol9c1 | DQ317662(I) | Portugal |
| O. volvulus isolate Ovol9c4 | DQ317665(I) | Portugal |
| O. volvulus isolate Ovol9c2 | DQ317663(I) | Portugal |
| O. gutturosa isolate Ogut2c5 | DQ317653(I) | Portugal |
| O. gutturosa isolate Ogut2c4 | DQ317652(I) | Portugal |
| O. linealis isolate Olien1c5 | DQ317657(I) | Portugal |
| O. volvulus | AF015193 (NAD2, CO1) | USA |
| Dirofilariaimmitis | AJ537512 (NAD2) | Australia:Victoria |
| Onchocercaflexuosa | AP017692(NAD2, CO1) | Miyazaki |
| Onchocercaochengi | AP017693(NAD2, CO1) | Miyazaki |
| Onchocercaochengi | AP017694(NAD2, CO1) | Miyazaki |
| Onchocerca volvulus | AP017695(NAD2, CO1) | Miyazaki |
| Loa loa | HQ186250 (NAD2) | USA |
| Onchocercaflexuosa | HQ214004 (NAD2, CO1) | USA |
| Dirofilariarepens | KR071802 (NAD2, CO1) | Germany |
| Onchocercavolvulus | KT599912 (NAD2, CO1) | Brazil |
| Onchocercaochengi isolate 1 | KX181289 (NAD2, CO1) | Cameroon |
| Onchocercaochengi isolate 2 | KX181290 (NAD2, CO1) | Cameroon |
| Dirofilariarepens | KX265047 (NAD2, CO1) | Italy |
| Dirofilariarepens voucher AW | KX265048 (NAD2, CO1) | Italy |
| Dirofilariarepens voucher S8 | KX265049 (NAD2, CO1) | Croatia |
| Dirofilaria sp. 'hongkongensis' | KX265050 (NAD2) | India |
| Wuchereriabancrofti | AP017705 (CO1) | Miyazaki |
| Wuchereriabancrofti | JN367461 (CO1) | USA |
| Wuchereriabancrofti | HQ184469 (CO1) | USA |
| Brugiatimori | AP017686 (CO1) | Miyazaki |
| Brugiamalayi | AF538716 (CO1) | UK |
| Brugiapahangi | AP017680 (CO1) | Miyazaki |
(I) = accession number of internal transcribed spacer subunit 2 (ITS2)–28S ribosomal RNA (28S rRNA); (COI) = accession number of cytochrome C oxidase subunit 1 (COI); (NAD2) = accession number of NADH dehydrogenase subunit 2 gene
Results
Gross, morphological and histopathological results
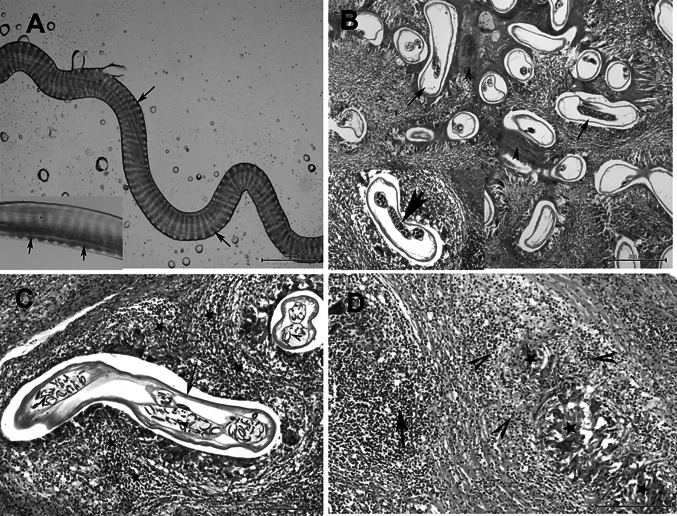
Round, flat, ovoid or irregular nodules containing Onchocerca were found in subcutaneous tissues of the back, and flanks of examined deer either in isolated or fused form, giving the appearance of lobulated nodules (cauliflower growth). They were solid and hard, and often surrounded by thick fibrous connective tissue. Microscope-aided gross and parasitological examination of all collected subcutaneous nodules revealed presence of very thin, long, nematodes identified as filarial worms. The living worms were transparent and opalescent, yellowish-white filarioids. The esophagus is divided into anterior muscular part and slightly longer posterior glandular part. The length of the females was much longer (about 80 mm) than the males (about 60 mm). The female worms had a rounded anterior ends and its widest diameter was approximately 170 -280 µm. The female cuticle was approximately 9 µm thick with external delicate transverse cuticular ridges with small protuberances (Fig. 1a). The males had a distinctive coiled posterior end with caudal alae and reduced number of post-cloacal caudal papillae with two unequal spicules (the left one is longer). The right spicule structure has a prominently dorsal hook. The transverse ridges were 4 µm apart at mid-body and tapering toward both ends.
Fig. 1.
a A fragment of the worm dissected out of the nodule showing marked and straight transverse ridges on the outer surface of the cuticle with small protuberances on the both lateral sides (arrow), Bar = 100 μm. Inset picture, showing the ridges (arrow), Bar = 20 μm. b Histological section of the subcutaneous nodule showing multiple adult female worms of O. flexuosa (arrows) with few males, the parasites are surrounded by homogenous eosinophilic material; inset picture showing the engorged uterus with the parasite, H&E, Bar = 200 μm. c Onchocerca females (arrow) with numerous microfilariae in the uterus (arrow heads) and granulomatous reaction surrounding the parasite (star) by H&E staining. Bar = 100 μm. d Degenerated filarial parasites represented by homogenous eosinophilic material (star) and severe granulomatous reaction (arrow) of macrophages and lymphocytes and eosinophilia (arrowheads) by H&E staining. Bar = 100 μm
Histopathological features of H&E stained sections are illustrated in (Fig. 1b, c, d). The subcutaneous nodules exhibited multiple compartments or cavities containing numerous numbers of nematode sectioned at various orientations surrounded by fibrosing granulomatous tissue. Most of the nodules were inhabited by several females, with or without microfilariae, and fewer male worms lying in small cavities (Fig. 1b). The nematode body was formed of an outer layer of eosinophilic cuticle and a thick muscle layer. In the pseudocoelom of some worms, two engorged uteri and small-sized intestine were observed. Many developing microfilariae were observed in the uterine cavity (Fig. 1c). Eosinophil, lymphocyte, and plasma cell infiltration was observed in the fibrous nodules around the worms exhibiting strong fibroplasias leading to the formation of a parasitic granuloma (Fig. 1c, d). Amorphous eosinophilic material (Splendore-Hoeppli reaction) was observed around some female worms (Fig. 1b). Degenerated worms surrounded by granulomatous reaction consisting of remnants of worms, cell debris, eosinophils, macrophages, lymphocytes, and giant cells with a thick peripheral fibrous layer were frequently observed (Fig. 1d).
Detection of O. flexuosa by PCR
All collected subcutaneous nodules representing the 17 deer under investigation tested positive for cox1, ITS2–28S rRNA, and NAD2 genes segments by conventional PCR. The PCR amplicons from Onchocerca worms yielded around 600 bp for ITS2-28S rRNA, and 1000 bp for both cox1 and NAD2 (Supplementary Fig. 1). The PCR products were further sequenced for alignment with the reference filarial strains available on gene bank data base, and homology analyses as well as generation of phylogenetic tree were performed.
Homology analysis of nucleotide and deduced amino acid sequences
Homology analysis of the nucleotide sequences of the three filarial nematode genes cox1, ITS2–28S rRNA, and NAD2 as well as the deduced amino acid sequences of cox1, and NAD2 genes segments showed variable identity with the genes of different Onchocerca species available on the gene bank (Tables 2, 3, 4 and 5).
Table 3.
showing the Identity % and P-distance between the cox1 sequence of isolated strain (O. flexuosa, LC318284.1) and the available sequence of Onchocerca species
| O._flexuosa_LC318284.1_COX1 | Identity % | P-Distance |
|---|---|---|
| O._flexuosa_LC318284.1_COX1 | 100 | |
| O_.flexuosa_AP017692.1 | 93.959 | 0.014 |
| O._flexuosa_HQ214004.1 | 93.635 | 0.018 |
| O._skrjabini:AM749269.1 | 91.776 | 0.038 |
| O._skrjabini:AM749270.1 | 91.776 | 0.038 |
| O._dewittei_japonica:AB518692.1 | 89.676 | 0.047 |
| O._dewittei_japonica_AB518691.1 | 89.368 | 0.048 |
| O._dewittei_japonica:_AB518873.1 | 89.522 | 0.047 |
| O._takaokai: AB972359.1 | 89.68 | 0.045 |
| O._takaokai: AB972360.1 | 89.68 | 0.045 |
| O._sp._wild_boar_AB518693.1 | 89.368 | 0.045 |
| O._volvulus_KT599912.1 | 90.334 | 0.046 |
| O._volvulus_AF015193.1 | 90.308 | 0.045 |
| O._volvulus_:KC167355.1 | 89.985 | 0.045 |
| O._volvulus_AP017695.1 | 90.308 | 0.046 |
| O._ochengi_NC_031891.2 | 89.966 | 0.048 |
| O._ochengi_KX181289.2 | 89.966 | 0.048 |
| O._ochengi_AP017694.1 | 89.852 | 0.049 |
| O._ochengi_AP017693.1 | 89.966 | 0.048 |
| O._ochengi_KX181290.2 | 89.738 | 0.051 |
| O._ochengi:_KP760202.1 | 90.159 | 0.048 |
| O._ochengi_KC167358.1 | 89.831 | 0.048 |
| O._ochengi_KC167350.1 | 89.831 | 0.048 |
| O._ochengi_KC167351.1 | 89.676 | 0.048 |
| O._gutturosa_AJ271617.1 | 90.836 | 0.044 |
| O._gutturosa_KP760201.1 | 90.348 | 0.044 |
| O._jakutensis_KT001213.1 | 89.318 | 0.045 |
| O._suzukii:_KX853333.1 | 91.429 | 0.050 |
| O._fasciata_JQ316672.1 | 90.28 | 0.047 |
| O._fasciata_MG188678.1 | 90.04 | 0.048 |
| O._lienalis_voucher:_KX853326.1 | 91.905 | 0.039 |
| O._lienalis_voucher_KX853325.1 | 89.873 | 0.037 |
| O._lupi_KX132091.1 | 89.953 | 0.045 |
| O._lupi_JX183106.1 | 89.401 | 0.047 |
| O._lupi_HQ207644.1 | 89.522 | 0.046 |
| O._sp._KC167354.1 | 89.522 | 0.051 |
| O._sp._KC167352.1 | 89.676 | 0.050 |
| O._sp._KC167353.1 | 89.676 | 0.050 |
Table 4.
showing the Identity % and P-distance between the ITS2-28S sequence of isolated strain (O. flexuosa LC321994.1) and the available sequence of Onchocerca species
| O._flexuosa: LC321994.1 ( ITS2_28S gene) | Identity % | P-Distance |
|---|---|---|
| O._flexuosa:LC321994.1 | 100 | 0.000 |
| O._gibsoni:DQ317647.1 | 84.81 | 0.067 |
| O._ochengi:DQ317659.1 | 84.304 | 0.074 |
| O._ochengi:FM206482.1 | 84.051 | 0.067 |
| O._fasciata:JQ316671.1 | 83.291 | 0.046 |
| O._volvulus:DQ317664.1 | 84.091 | 0.074 |
| O._ochengi:DQ317660.1 | 83.333 | 0.074 |
| O._volvulus:AF228572.1, AF228567.1, AF228569.1 | 84.091 | 0.074 |
| O._volvulus:AF228566.1 | 83.838 | 0.074 |
| O._gutturosa:DQ317652.1 | 83.586 | 0.046 |
| O._gibsoni:DQ317646.1, DQ317641.1 | 82.785 | 0.067 |
| O._gibsoni:DQ317644.1 | 82.576 | 0.067 |
| O._gibsoni:DQ317640.1 | 82.532 | 0.067 |
| O._gutturosa:DQ317651.1 | 82.785 | 0.039 |
| O._sp. 1_WS-2017:MG192127.1 | 85.526 | 0.039 |
| O._gutturosa:DQ317650.1 | 80.759 | 0.046 |
| O._ochengi:DQ317661.1 | 83.333 | 0.074 |
| O._ochengi:DQ317658.1 | 82.828 | 0.060 |
| O._gutturosa: DQ317649.1 | 81.538 | 0.046 |
| O._dewittei_dewittei: MG192132.1, MG192131.1, MG192130.1, MG192129.1, MG192128.1, MG192133.1 | 83.013 | 0.097 |
| O._dewittei_japonica:MG192134.1_ | 84.722 | 0.074 |
| O._sp._1_WS-2017:MG192126.1 | 85.197 | 0.060 |
| O._sp._1_WS-2017:MG192125.1_ | 85.197 | 0.067 |
| O._linealis:DQ317655.1_ | 76.336 | 0.074 |
| O._linealis:DQ317654.1_ | 76.081 | 0.074 |
| D._repens:AY693808.1 | 68.646 | 0.171 |
| D._repens:JQ039744.1 | 68.585 | 0.187 |
| D._repens:JQ039743.1 | 69.83 | 0.187 |
| D._immitis:EU182330.1 | 66.75 | 0.172 |
| D._immitis:EU087699.1_ | 67.866 | 0.163 |
| O._linealis:DQ317656.1 | 76.031 | 0.074 |
| O._linealis:DQ317657.1 | 76.081 | 0.074 |
Table 5.
showing the Identity % and P-distance between the NAD2 sequence of isolated strain (O. flexuosa LC320669.1) and the available sequence of Onchocerca species
| O._flexuosa_LC320669.1_NAD2 | Identity % | P-Distance |
|---|---|---|
| O._flexuosa_ LC320669.1 | 100 | |
| O._flexuosa AP017692.1_ | 90.811 | 0.107 |
| O._flexuosa HQ214004.1 | 90.45 | 0.113 |
| O._volvulus AF015193.1 | 86.876 | 0.167 |
| O._volvulus AP017695.1 | 86.691 | 0.170 |
| O._volvulus KT599912.1 | 86.691 | 0.170 |
| O._ochengi KX181290.2 | 85.661 | 0.185 |
| O._ochengi AP017693.1_ | 86.406 | 0.172 |
| O._ochengi AP017694.1 | 86.406 | 0.172 |
| O._ochengi NC_031891.2 | 86.406 | 0.172 |
| O._ochengi KX181289.2 | 86.406 | 0.172 |
| D._sp._hongkong. KX265050.1 | 86.1 | 0.202 |
| D._immitis AJ537512.1_ | 83.012 | 0.293 |
| Loa_loa HQ186250.1_ | 83.721 | 0.273 |
| D._repens KX265047.1_ | 87.259 | 0.176 |
| D._repens KR071802.1_ | 87.259 | 0.176 |
| D._repens KX265049.1 | 87.066 | 0.180 |
| D._repens KX265048.1_ | 87.452 | 0.172 |
| S._digitata KY284626.1_ | 82.171 | 0.374 |
| S._digitata GU138699.1 | 81.977 | 0.394 |
| A._viteae AP017679.1_ | 82.659 | 0.354 |
| A._viteae HQ186249.1_ | 82.659 | 0.354 |
| W._bancrofti JF775522.1_ | 82.398 | 0.359 |
| W._bancrofti AP017705.1_ | 82.398 | 0.359 |
| W._bancrofti JN367461.1_ | 82.205 | 0.364 |
| W._bancrofti JQ316200.1_ | 82.012 | 0.377 |
| W._bancrofti HQ184469.1_ | 82.205 | 0.364 |
| B._pahangi AP017680.1_ | 81.783 | 0.343 |
| B._timori AP017686.1 | 81.008 | 0.370 |
Alignment analysis of the nucleotide and amino acid sequences of O. flexuosa of our isolates and other published filarial worms was done. The results of alignment analysis of the nucleotide sequences of O. flexuosa of our isolates revealed presence of variation (mutations, insertions, and/or deletions) at several positions. Some of these nucleotide mutations were transcribed, resulting in subsequent mutation of the deduced amino acids, whereas others were silent nucleotide mutations. The nucleotide sequences of ITS2–28S rRNA gene segments from examined deer showed that the DNA fragment exhibited highest similarities with the sequences of some Onchocerca species (O. gibsoni, O. ochengi, O. volvulus, and O. gutturosa); data for which is available in GenBank.
The obtained nucleotide sequences of cox1 of the O. flexuosa isolate were compared with those of other 19 reference filarial strains. The results displayed regularity in variation at several positions as shown in (Supplementary Fig. 2). On the same side, the alignment analysis of the obtained nucleotide sequences of ITS2–28S rRNA with other reference filarial strains revealed presence of variation at several positions (Supplementary Fig. 3). In addition, the obtained nucleotide sequences of NAD2 of the O. flexuosa isolate were compared with those of other reported filarial strains. The results displayed regularity in the mutations at positions (Supplementary Fig. 4).
We compared the nucleotide sequences of Cox1, ITS2–28S rRNA and NAD2 genes sequences of Cox1 and ITS2–28S rRNA genes of our samples from captured deer with those of all Onchocerca species available in GenBank. Cox1 sequence of this study showed high identity (94%) with O. flexuosa isolated from Miyazaki Prefecture in Japan and from USA red deer isolates. It also showed 90–91% identity with human O. volvulus isolate obtained from Brazil, cattle O. ochengi isolate obtained from Cameroon, O. ochengi obtained from Miyazaki Prefecture Japan, The novel sequences obtained in the present study also exhibited reduced identity (89.37%-89.68%, 91.78%) with Onchocerca dewittei japonica and Onchocerca skrjabini respectively using ITS2_28S sequence, and (87–89%) with human filarial worms (Wuchereria bancrofti, Brugia timori, B. malayi, and B. pahangi) detected by using NAD2 sequence.
As shown in (Table 3), p-distances in nucleotide sequences of cox1 were 0–0.044 for intra-species and 0.038–0.051 for inter-species of Onchocerca. Similarly the results of p-distances in nucleotide sequences of ITS2–28S rRNA and NAD2 genes were showed in tables (3, 4) and (supplementary tables 1.2 and 3).
Phylogenetic analyses
Phylogenetic trees for Onchocerca spp were constructed using the NJ and ML methods for the nucleotide sequences of cox1, NAD2, and ITS2–28S rRNA genes (Figs. 2, 3, and 4).
Fig. 2.
Phylogeny of Onchocerca spp based on GenBank sequences of the cox1. There were a total of 576 positions in the final dataset. Notice: the present isolate, O. flexuosa, formed a monophyletic subgroup sharing the same subclade with O. flexuosa isolated from Miyazaki Prefecture and USA, which appeared as sisters in the same branch
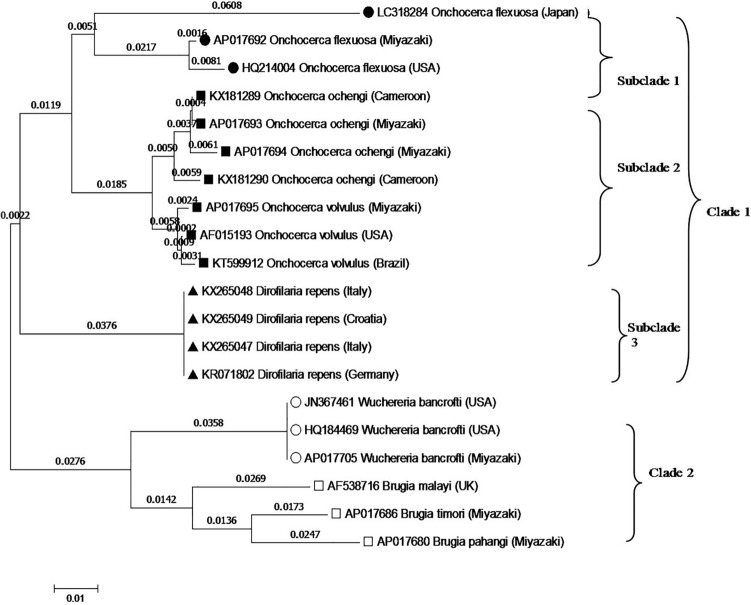
Fig. 3.
Phylogeny of filariae and related nematodes based on ITS2 taxa. The GenBank accession numbers for each sequence are shown adjacent to each strain
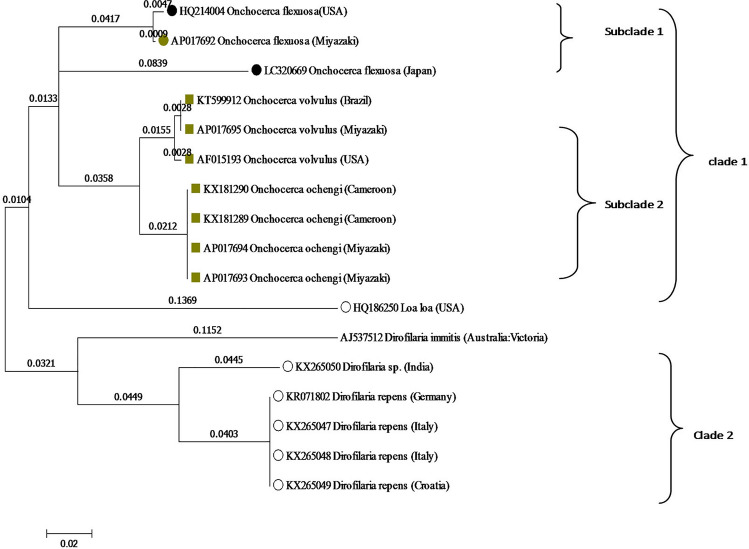
Fig. 4.
Evolutionary relationships of NAD2 taxa. The analysis involved 30 nucleotide sequences. Codon positions included were 1st + 2nd + 3rd + Noncoding
ITS2–28S rRNA region
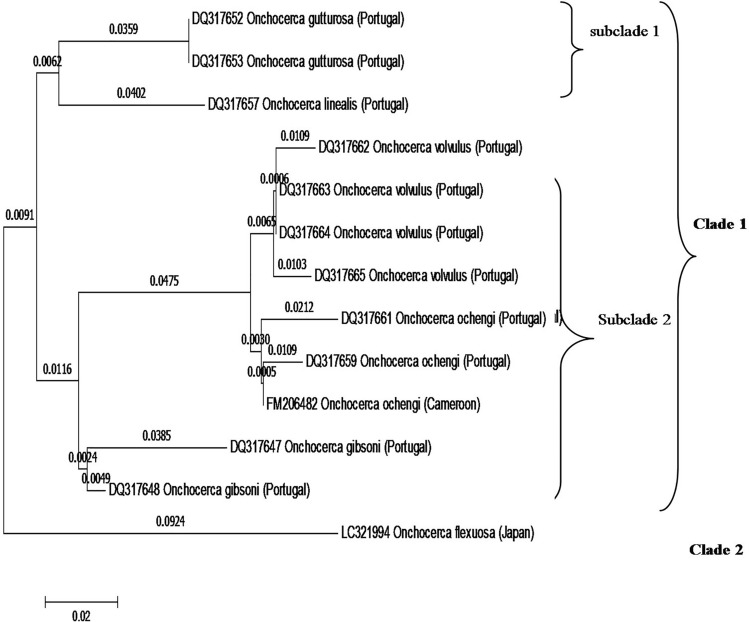
In the same scene, the phylogenetic tree generated based upon the ITS2–28S rRNA region as shown in (Fig. 2), revealed grouping of the onchocerca spp into two clades, clade 1 divided into two subclades. The first subclade included O. gutturosa and O. linealis. The one second containing O. volvulus (four leaves), O. ochengi from Porugal and Cameron. Also, give a relation with O. gibsoni. Clade 2 included O. flexuosa from Japan.
Cox1 gene
The phylogenetic tree generated based upon cox1 gene sequences as shown in (Fig. 3), showed that, the filarial species were distributed in two main clades. The first clade included three subclades the first one include O. flexuosa of different countries and districts (Japan, Miyazaki Prefecture and USA). The second subclade including O. ochengi isolated from Cameron and Miyazaki Prefecture. Also, related to O. volvulus (three leaves). The third subclade related to D. repens (four leaves). Clade II make a relation with W. bancrofti and Brugia sp.
NAD2 region
Similar to the phylogenetic tree generated based upon the Cox1 region; the phylogenetic tree of NAD2 region revealed presence of two clades. Clade I was divided into two subcldes. First subclade included only O. flexuosa species. Second Subclade included O. volvulus (three leaves), O. ochengi (four leaves) and give a relation with Loa loa. Clade II showing the relation with Dirofilaria species (Fig. 4).
The results clearly identified that Onchocerca species is the cause of parasitic nodules in the deer under investigation in the present study. As shown in (Table 3, 4, and 5), p-distances in nucleotide sequences of cox1 were 0–0.018 for intra-species and (0.017–0.051, 0.017–0.117 for inter-species of Onchocerca. In the same scene, our phylogenetic analysis based upon cox1, NAD2 and ITS2–28S rRNA genes indicated a close affinity between the present isolate of O. flexuosa and the previously isolated O. flexuosa, O. skrjabini, and O. eberhardi, isolated from Sika deer or serows in Japan. In contrast, Onchocerca flexuosa was distant from O. takaokai n. sp and O. dewittei japonica, as shown in (Figs. 2–4).
Discussion
Filariasis, and anthroponotic onchocerciasis have been reported worldwide and have caused problems either in animals or humans (Krueger et al. 2007; Uni et al. 2010).
In the current study, parasitic nodules in the form of yellowish subcutaneous nodules located at the back, mainly at the sacrum, lumbar region and flanks and rarely on the lateral abdomen or proximal parts of the extremities, in addition, few scattered nodules were located at the forelimbs and neck in Japanese Sika deer (Cervus nippon) captured from Gifu and Shiga Prefectures in Japan. These findings are in agreement with the previously reported onchocerciasis (Plenge-Bönig et al. 1995). The morphology of the present filarioids have the main characteristics of the genus Onchocerca that given by Bain (Bain 1981) i.e. females have a thickened cuticle with transverse annular ridges on their surface and weakly developed muscular layer.
Histopathological and the measurements analyzed, matched with those given in the description of O. flexuosa, carried out by earlier investigators (Bain and Schulz-Key 1974; Hidalgo-Argüello et al. 2010; Hidalgo et al. 2015). Chronic parasitism in the form of multiple granulations around the worm and marked leukocytic cell infiltration of eosinophils, lymphocytes, macrophages, and plasma cells were observed and these granulomata were found mainly around female worms. Presences of many degenerated worms within several cavities in the nodules are an indication of a strong immunological response against O. flexuosa (Hidalgo et al. 2015; Suzuki and UNI S, KOMATSU T, YAMAMOTO Y, ATOJI Y, 1997). Female worms were found more frequently in the nodules than males, they were embedded in the nodular fibrous tissue, and most of them were usually immobile due to an early degenerative alteration of their muscles. In contrast, mobility activity of males is higher than females as they can migrate subcutaneously leaving the nodules. Similar conclusions have been reached before (Hidalgo et al. 2015; Franz et al. 1987; Plenge-Bo¨nig A, Kro¨mer, M.&Bu¨ ttner, D.W. 1995).
The presence of rivers and mountain streams enhance the vectors survival; resulting in high prevalence and intensity (Hidalgo et al. 2015; Franz et al. 1987; Plenge-Bo¨nig A, Kro¨mer, M.&Bu¨ ttner, D.W. 1995). Both temperature and moisture are the most important environmental changes for all organisms. So, the ecology and the epidemiology of O. flexuosa are differing according the location. The prevalence of O. flexuosa in Sika deer was unknown in the Japan especially in the above mentioned examined regions. Prevalence of Onchocerca spp in wild and domesticated animals had previously appeared to be restricted to central and eastern Japan in Myazaki city (unpublished manuscript, gene bank reference number at https://www.ncbi.nlm.nih.gov/nuccore/AP017692.1), perhaps because these were the only areas where they had been studied. To the best of our knowledge, this is the first record of O. flexuosa in that area, as there is only one record of O. flexuosa from Myazaki/Japan in the GenBank.
In the present study, in order to confirm the molecular identity of O. flexuosa and to investigate its phylogenetic position, we compared the nucleotide sequences of cox1, NAD2 and ITS2–28S rRNA with those of all Onchocerca species available in GenBank. The nucleotide sequence of cox1 in our samples of O. flexuosa was identical to other O. flexuosa isolated from Japan and was similar (0.2% nucleotide differences) to O. skrjabini O. boehmi and O. lupi. The present isolate of O. flexuosa differed from O. dewittei japonica and O. takaokai n. sp with differences of 4.7–4.5% respectively in the nucleotide sequences of cox1. Sequence analyses of the ITS2–28S rRNA gene of O. flexuosa revealed a 5.8 –7.4% difference compared with the nucleotide sequences of O. dewittei japonica and a 4.8– 8.5% difference compared with that of other congeners. Similarly, the ND2 sequence analysis revealed a 5.8 –7.4% nucleotide sequences difference compared with that of O. dewittei japonica and a 4.8– 8.5% difference compared with that of other congeners.
P-distances in nucleotide sequences of examined genes as mentioned in the results and our phylogenetic analysis based upon cox1, NAD2 and ITS2–28S rRNA genes indicated a close affinity between the present isolate of O. flexuosa and the previously isolated O. flexuosa, O. skrjabini, and O. eberhardi, isolated from Sika deer or serows in Japan. In contrast, O. flexuosa was distant from O. takaokai n. sp and O. dewittei japonica. Moreover, our results confirmed the previous reports (Yamaguti 1963; Anderson et al. 1976), indicated that the phylogeny of the family Onchocercidae based on the previously mentioned genes sequences plays a vital role in the classification of filarial worms along with the classification based upon morphological characters.
In a previous studies, DNA analysis and barcoding based on cox1 and other mitochondrial genes was considered a reliable method for species identification of filarioid nematodes, with a high coherence with classical taxonomy (Ferri et al. 2009). In recent studies different approaches to species recognition generated similar results (Miller 2007), which suggested that integration of traditional and molecular approaches to species recognition is possible (Dettman et al. 2003). More recently identification of Onchocerca spp using cox1 as a molecular marker is considered accurate as previously indicated for Onchocerca spp (Lefoulon et al. 2017), as well as other filarial species (Ferri et al. 2009). DNA analysis was considered a good and reliable approach for taxonomical identification of filarioid nematodes, with a high coherence with classical taxonomy (Ferri et al. 2009).
In the present study, the filarial parasite was identified as O. flexuosa based on traditional and molecular analysis. Phylogenetic analysis based on cox1, ITS2–28S rRNA, and NAD2 gene sequences revealed that only three main groupings were recorded in the family Onchocercidae, viz., Onchocerca spp, Dirofilaria spp, and Brugia + Wuchereria. Similar conclusion has been reached by Yatawara et al. (2007); Huang et al. 2009) based upon molecular characteristics and phylogeny of the Filarioidea superfamily. Thus, the phylogenetic aspects of filarial worms may be resolved by analysis based on cox1, ITS2–28S rRNA, and NAD2 genes.
In our opinion, the light of the present work suggests that cox1, ITS2–28S rRNA, and NAD2 appeared an appropriate molecular marker for identification of filarioid nematodes up to species level. Similar suggestion has been reached by using cox1 and 12S rDNA genes for identification of filarial nematodes up to species level (Ferri et al. 2009). The phylogenetic analyses of cox1, the ITS2–28S rRNA, and NAD2 genes in that study indicated that O. flexuosa isolated from Japanese Sika deer was identical to Onchocerca spp in terms of nucleotide sequences. According to Ferri et al. (2009), no overlap of intra- and interspecific nucleotide divergence at the cox1 gene occurs at distance values lesser than threshold values (approximately 4.8%). Based upon intra- and interspecific nucleotide divergences, in this study we calculated the genetic distance between cox1 from two different species, and it was lesser than the threshold value of 4.8%. Therefore, our findings clearly document the conclusion that the present isolate is O. flexuosa, which is distinguished from O. dewittei japonica, O. takaokai and other congeneric species at the species level, and has a close affinity to other O. flexuosa previously reported.
The sequencing analysis is of great importance to identify the origin of the genus Onchocerca and its relation with other members of the family and this characteristic could be used to medicinally control the infection by targeting a specific gene. The emergence of more infectious diseases is a significant problem for the world economy and public health. Majority of these diseases originate in wildlife and spread to other species including animals and humans, and their incidence has increased significantly over the past few decades (Jones et al. 2008). Thus, disease surveillance efforts and accurate diagnosis should be applied frequently especially in wildlife to control spread of these pathogens to human or other species.
Supplementary Information
Below is the link to the electronic supplementary material.
72
Declarations
Conflicts of interest
No potential conflict of interest was reported by the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abd-Ellatieff HA, Abou Rawash AA, Ellakany HF, Goda WM, Suzuki T, et al. Molecular characterization and phylogenetic analysis of a virulent Marek’s disease virus field strain in broiler chickens in Japan. Avian Pathol. 2018;47:47–57. doi: 10.1080/03079457.2017.1362497. [DOI] [PubMed] [Google Scholar]
- Anderson RC (2000) Nematode parasites of vertebrates: their development and transmission: Cabi
- Anderson RC, Bain O. Keys to genera of the order Spirurida. Part 3. Diplotriaenoidea, Aproctoidea and Filarioidea. In: Anderson RC, Chabaud AG, Willmott S, editors. CIH Keys to the Nematode Parasites of Vertebrates. Farnham Royal: Commonwealth Agricultural Bureaux; 1976. pp. 59–116. [Google Scholar]
- Anderson RC, Chabaud AG, Willmott S (2009) Keys to the nematode parasites of vertebrates: archival volume: CABI.
- Bain O. Le genre Onchocerca: hypothèses sur son évolution et clé dichotomique des espèces. Ann Parasitol Hum Comp. 1981;56:503–526. doi: 10.1051/parasite/1981565503. [DOI] [PubMed] [Google Scholar]
- Bain O (2002). Evolutionary relationships among filarial nematodes. in: Chabaud, A.G., Bain, O., 1976. La ligne´e Dipetalonema. Nouvel essai de classification. Ann Parasitol Hum Comp. 51, 365–397 [PubMed]
- Bain O, Schulz-Key H. The species of Onchocerca in the red deer: redescription of O. flexuosa (Wedl, 1856) and description of O. tubingensis n. sp. and O. tarsicola n. sp (author's transl) Tropenmed Parasit. 1974;25:437–449. [PubMed] [Google Scholar]
- Bain O, Schulz-Key (1974) Les Onchocerques du Cerf européen: Redescription d'O. flexuosa (Wedl, 1856) et description d'O. tubingensis n. sp. et O. tarsicola n. sp. [PubMed]
- Boijsen B, Uhlhorn H, Ågren E, Höglund J. Nodular onchocercosis in red deer (Cervus elaphus) in Sweden. Int. J. Parasitol. Parasit. Wildlife. 2017;6:340–343. doi: 10.1016/j.ijppaw.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantas-Torres F, Otranto D. Dirofilariosis in the Americas: a more virulent dirofilaria immitis? Parasit Vectors. 2013;6:1–9. doi: 10.1186/1756-3305-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettman JR, Jacobson DJ, Turner E, Pringle A, Taylor JW. Reproductive isolation and phylogenetic divergence in Neurospora: comparing methods of species recognition in a model eukaryote. Evolution. 2003;57:2721–2741. doi: 10.1111/j.0014-3820.2003.tb01515.x. [DOI] [PubMed] [Google Scholar]
- Ferri E, Barbuto M, Bain O, Galimberti A, Uni S, et al. Integrated taxonomy: traditional approach and DNA barcoding for the identification of filarioid worms and related parasites (Nematoda) Front Zool. 2009;6:1. doi: 10.1186/1742-9994-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd R, Abebe E, Papert A, Blaxter M. Molecular barcodes for soil nematode identification. Mol Ecol. 2002;11:839–850. doi: 10.1046/j.1365-294X.2002.01485.x. [DOI] [PubMed] [Google Scholar]
- Franz M, Schulz-Key H, Copeman DB. Electron-microscopic observations on the female worms of six Onchocerca species from cattle and red deer. Parasitol Res. 1987;74:73–83. doi: 10.1007/BF00534936. [DOI] [PubMed] [Google Scholar]
- Genchi C, Kramer L, Rivasi F (2011) Dirofilarial infections in Europe. Vector borne and zoonotic disease. Lachmot, NY [DOI] [PubMed]
- Hidalgo M, Martínez A, Carreno R, González S, Ferreras M, et al. Levels of infection, pathology and nodule size of Onchocerca flexuosa (Nematoda: Onchocercidae) in red deer (Cervus elaphus) from northern Spain. J Helminthol. 2015;89:326–334. doi: 10.1017/S0022149X1400011X. [DOI] [PubMed] [Google Scholar]
- Hidalgo-Argüello M, Díez-Baños N, Martínez-Delgado A (2010) Parasitic infections in red deer and their repercusions on animal and human health: a study in the North of the province of León. Parasitic Diseases of Wild Animals and Sustainable Environment The Wild/domestic Interface Universidad Complutense de Madrid, Área de Ciencias de la Salud: 63–95.
- Huang H, Wang T, Yang G, Zhang Z, Wang C, et al. Molecular characterization and phylogenetic analysis of Dirofilaria immitis of China based on COI and 12S rDNA genes. Vet Parasitol. 2009;160:175–179. doi: 10.1016/j.vetpar.2008.10.053. [DOI] [PubMed] [Google Scholar]
- Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, et al. Global trends in emerging infectious diseases. Nature. 2008;451:990. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger A, Fischer P, Morales-Hojas R. Molecular phylogeny of the filaria genus Onchocerca with special emphasis on Afrotropical human and bovine parasites. Acta Trop. 2007;101:1–14. doi: 10.1016/j.actatropica.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Lefoulon E, Giannelli A, Makepeace BL, Mutafchiev Y, Townson S, et al. Whence river blindness? The domestication of mammals and host-parasite co-evolution in the nematode genus Onchocerca. Int J Parasitol. 2017;47:457–470. doi: 10.1016/j.ijpara.2016.12.009. [DOI] [PubMed] [Google Scholar]
- Markmann M, Tautz D. Reverse taxonomy: an approach towards determining the diversity of meiobenthic organisms based on ribosomal RNA signature sequences. Philosoph. Transact. Royal Soc. London b: Biolog. Sci. 2005;360:1917–1924. doi: 10.1098/rstb.2005.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFrederick QS, Haselkorn TS, Verocai GG, Jaenike J. Cryptic Onchocerca species infecting North American cervids, with implications for the evolutionary history of host associations in Onchocerca. Parasitology. 2013;140:1201–1210. doi: 10.1017/S0031182012001758. [DOI] [PubMed] [Google Scholar]
- Miller SE. DNA barcoding and the renaissance of taxonomy. Proc Natl Acad Sci. 2007;104:4775–4776. doi: 10.1073/pnas.0700466104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan MT, Balke M, Gregory TR, Vogler AP. DNA-based species delineation in tropical beetles using mitochondrial and nuclear markers. Philosoph. Transact. Royal Soc. London b: Biolog. Sci. 2005;360:1925–1933. doi: 10.1098/rstb.2005.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Hojas R, Cheke RA, Post R. Molecular systematics of five Onchocerca species (Nematoda: Filarioidea) including the human parasite, O. volvulus, suggest sympatric speciation. J Helminthol. 2006;80:281–290. [PubMed] [Google Scholar]
- Morchón R, Carretón E, González Miguel J, Mellado Hernández I. Heartworm disease (Dirofilaria immitis) and their vectors in Europe–new distribution trends. Front Physiol. 2012;3:196. doi: 10.3389/fphys.2012.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otranto D, Eberhard ML. Zoonotic helminths affecting the human eye. Parasit Vect. 2011;4:1–21. doi: 10.1186/1756-3305-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plenge-Bo¨nig A, Kro¨mer, M.&Bu¨ ttner, D.W. Light and electronmicroscopy studies onOnchocerca jakutensis and O. flexuosa of red deer show different host-parasite interactions. Parasitol Res. 1995;81:66–73. doi: 10.1007/BF00932419. [DOI] [PubMed] [Google Scholar]
- Plenge-Bönig A, Krömer M, Büttner D. Light and electron microscopy studies on Onchocerca jakutensis and O. flexuosa of red deer show different host-parasite interactions. Parasitol Res. 1995;81:66–73. doi: 10.1007/BF00932419. [DOI] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Y Suzuki Uni S, Komatsu T, Yamamoto Y, Atoji Y, 1997 Olecranon lesions caused by Onchocerca skrjabini in wild Japanese serows (Capricornis crispus) J Vet Med Sci 59 387 390 [DOI] [PubMed]
- Uni S, Bain O, Takaoka H, Katsumi A, Fujita H, et al. Diversification of Cercopithifilaria species (Nematoda: Filarioidea) in Japanese wild ruminants with description of two new species. Parasite-Paris. 2002;9:293–304. doi: 10.1051/parasite/2002094293. [DOI] [PubMed] [Google Scholar]
- Uni S, Boda T, Daisaku K, Ikura Y, Maruyama H, et al. Zoonotic filariasis caused by Onchocerca dewittei japonica in a resident of Hiroshima Prefecture, Honshu, Japan. Parasitol Int. 2010;59:477–480. doi: 10.1016/j.parint.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Yamaguti S. Systema Helminthum. Inc, New york: The Nematodes of Vertebrates. Interscience Publ; 1963. [Google Scholar]
- Yatawara L, Wickramasinghe S, Nagataki M, Rajapakse R, Agatsuma T. Molecular characterization and phylogenetic analysis of setaria digitata of Sri Lanka based on CO1 and 12S rDNA genes. Vet Parasitol. 2007;148:161–165. doi: 10.1016/j.vetpar.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Yatawara L, Wickramasinghe S, Rajapakse R, Agatsuma T. The complete mitochondrial genome of Setaria digitata (Nematoda: Filarioidea): Mitochondrial gene content, arrangement and composition compared with other nematodes. Mol Biochem Parasitol. 2010;173:32–38. doi: 10.1016/j.molbiopara.2010.05.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.